Scientists have long recognized ketones and esters as vital components in chemical synthesis, particularly within the pharmaceutical industry. These molecules, each serving as building blocks for a variety of drugs, hold immense promise but also come with substantial limitations. Ketones and esters are both versatile, present in numerous chemical processes, but traditionally their structures have been resistant to change. With their high stability, altering the molecular makeup of ketones and esters was a significant challenge for chemists. This limitation stems primarily from the resilience of their carbon-hydrogen (C-H) bonds—bonds that, while integral to the structure, make ketones and esters relatively inert to catalysts, chemicals that speed up reactions by altering the molecular structures.
However, a pioneering discovery from researchers at Scripps Research, published in Nature on January 8, could very well unlock these previously untapped areas of chemistry. The innovative method promises to streamline the use of ketones and esters in chemical reactions, making them more adaptable for synthesis without the typical requirements for multiple preparatory steps. These groundbreaking findings stand to have significant consequences for drug development and beyond, marking a significant advancement toward more efficient and sustainable chemical production.
Senior author Dr. Jin-Quan Yu, a prominent figure in the study of chemical synthesis, emphasizes the importance of ketones in creating new drug molecules. Ketones are foundational in chemical synthesis processes, which involve constructing complex molecular structures from simpler ones. By breaking down previously impenetrable chemical barriers within ketones and esters, Yu and his colleagues have simplified the pathway to modify these substances, furthering the potential for pharmaceutical applications. The ability to manipulate these molecules more effectively could accelerate the development of life-changing medications, making synthesis faster and potentially lowering production costs.
For over a decade, Dr. Yu and his lab have been instrumental in refining a technique known as C-H activation—a catalytic method that enables chemists to bypass the obstacles of C-H bonds in otherwise inert molecules. This technique plays a crucial role in unlocking new sites of reactivity in organic compounds. While the strategy has already shown success with molecules such as alcohols, acids, and amines, extending it to ketones and esters represents the completion of an ambitious journey to extend this approach to all critical classes of organic molecules used in drug synthesis.
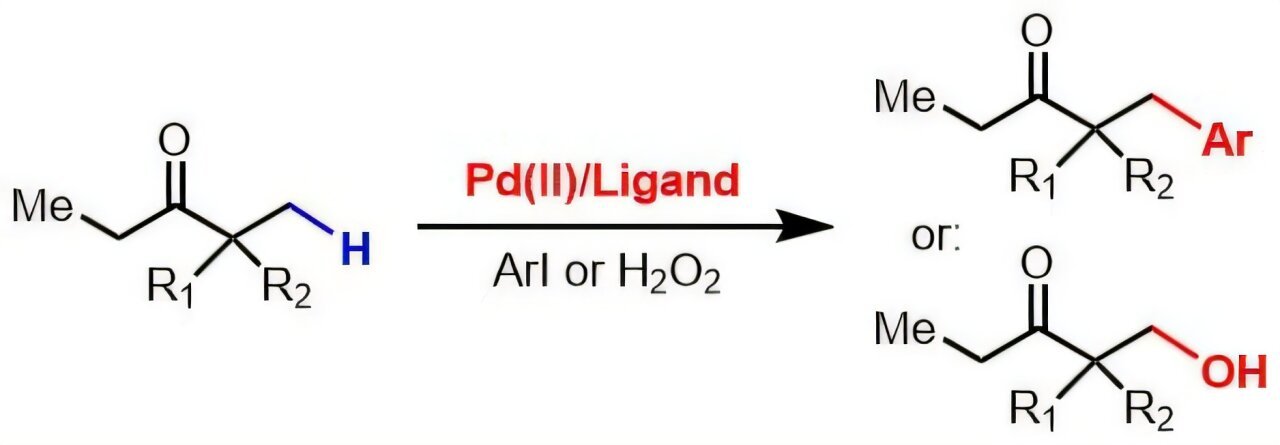
Ketones and esters, while important in drug synthesis, have historically been resistant to the benefits of catalysis. Part of the challenge lies in the chemical bonding of the C-H bonds, which are inherently strong and stable. These bonds naturally impede reaction with catalysts, often requiring a more involved, multi-step approach to create the desired modifications. The difficulty increases because ketones and esters have a low affinity for the metal catalysts traditionally used in C-H activation, limiting their potential for chemical alteration. This issue presented a problem in designing effective chemical transformations using these compounds without the addition of external chemical directing groups—additional chemicals designed to help guide catalysts. These extra steps not only increase the time and complexity of synthesis but can also lead to additional waste, an outcome increasingly at odds with the broader move towards green chemistry and sustainable chemical practices.
Dr. Yu’s team overcame this significant hurdle with a clever innovation: a newly developed catalyst system that integrates a special molecule called monoprotected amino neutral amide ligand. This molecule aids in binding palladium—a widely used catalyst—more effectively to ketones and esters, overcoming the inert nature of their chemical bonds. The success of the catalyst is further enhanced by using tetrafluoroboric acid, a strong acid that stabilizes the catalytic system long enough to enable the transformation of ketones and esters by breaking their C-H bonds. By leveraging this novel approach, the researchers successfully induced reactions such as arylation and hydroxylation, both of which are crucial for adding new functional building blocks to molecules. This expansion of chemical tools allows scientists to create highly specialized and biologically relevant molecules, with fewer steps and less chemical waste.
The broader impact of this method is vast. By unlocking new possibilities for modifying ketones and esters, the technique offers several advantages for industrial and scientific applications, particularly in the field of pharmaceutical manufacturing. The process allows for faster, more affordable production of complex drug molecules—an outcome that could be crucial for meeting global healthcare needs. At the same time, it supports sustainability in the production process by reducing waste and supporting the goals of green chemistry. The broader benefits, however, extend beyond medicine. Ketones and esters play essential roles not just in pharmaceuticals, but also in fields ranging from materials science to agriculture. Ketones are used in manufacturing products like durable resins, solvents, and even plastics, while esters are vital ingredients in fragrances, biodegradable plastics, and agrochemical formulations.
One area with great potential in Yu’s future work is the creation of chiral molecules. Chirality refers to molecules that are mirror images of each other yet cannot be superimposed on one another. This characteristic is critical in producing many pharmaceutical products, as different chiral forms of a molecule can have dramatically different effects on the human body. By expanding his catalytic system to facilitate the creation of chiral molecules, Dr. Yu could significantly enhance pharmaceutical research, ensuring that molecules are not just structurally precise but also biologically functional.
Through his innovative work on C-H activation in ketones and esters, Dr. Yu’s research opens the door to new possibilities in chemical synthesis, one that marries scientific precision with broad-ranging applicability. As the world continues to face challenges related to health, sustainability, and innovation, advances like these provide much-needed solutions, ensuring that the synthesis of crucial compounds—whether for medicine, materials science, or the environment—is more efficient, environmentally friendly, and sustainable. This research may well serve as the gateway to new generations of important drugs, chemicals, and materials, effectively advancing many industries by making chemical processes faster and greener.
Reference: Yi-Hao Li et al, β-C−H bond functionalization of ketones and esters by cationic Pd complexes, Nature (2025). DOI: 10.1038/s41586-024-08281-4