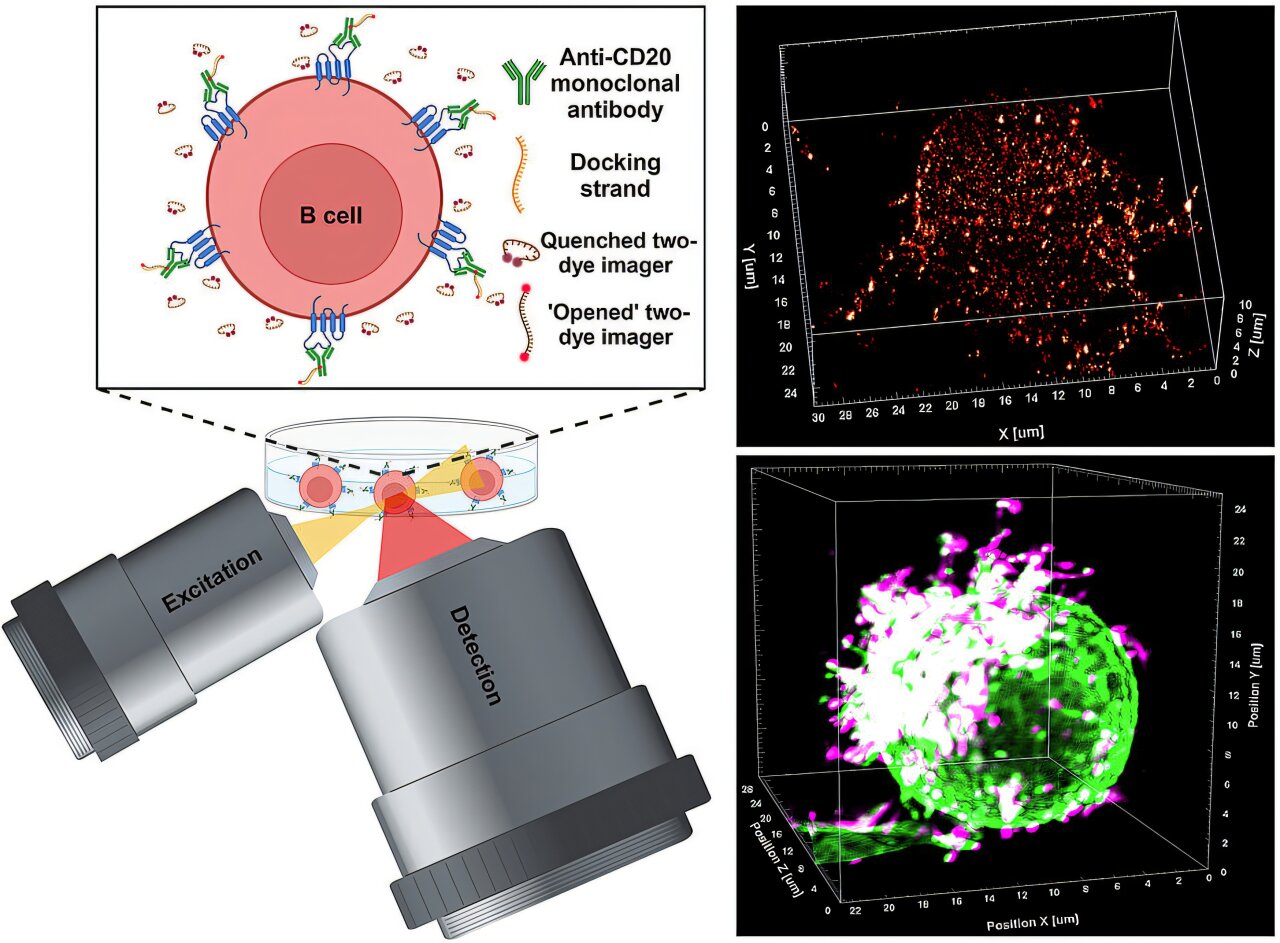
Chronic lymphocytic leukemia (CLL) is a type of blood cancer characterized by the uncontrollable growth of B cells, which are a vital part of the immune system. These cells proliferate abnormally and crowd out healthy blood cells, leading to the weakening of the immune system. A prominent approach for treating blood cancers like CLL involves using therapeutic antibodies that target and bind to a specific protein on the surface of B cells—known as CD20. This interaction triggers a series of immune reactions that result in the destruction of the cancerous cells. While antibody-based therapies have been employed for around 30 years, much about the precise mechanisms behind these therapies remained unclear—until recently.
One of the major questions that persisted in the field was understanding how antibodies bind to the CD20 protein on tumor cells, particularly B cells, and how this triggers the subsequent immune response that leads to cancer cell destruction. In a groundbreaking development, a team of researchers led by Professor Markus Sauer, a biophysicist from the Biocenter at Julius-Maximilians-Universität (JMU) Würzburg, Germany, has devised a novel super-resolution microscopy method. This technology offers unprecedented insights into the binding interactions of therapeutic antibodies with CD20 molecules on B cell surfaces, enabling observations at the molecular level in 3D. Their findings promise to change the way we understand these therapies, contributing directly to the enhancement of cancer treatments in the future.
The advancements, published in the prestigious journal Science, provide the first direct observations of therapeutic antibodies in action against tumor cells. The new super-resolution microscopy method, referred to as LLS-TDI-DNA-PAINT, allows for incredibly detailed analysis of interactions between antibodies and their target molecules on tumor cells. This tool is crucial in not only understanding the exact mechanics of how antibodies work against cancer but also in tracking their effectiveness. By gaining this detailed understanding, scientists and clinicians can develop better, more targeted therapies, ultimately improving patient outcomes in cancer treatments.
To conduct the research, the team employed the LLS-TDI-DNA-PAINT method on fixed and live Raji B cells. These cells, originating from a patient with Burkitt’s lymphoma, are commonly used for cancer studies. The research team introduced the cells to four different therapeutic antibodies: RTX (Rituximab), OFA (Ofatumumab), OBZ (Obinutuzumab), and 2H7, each of which is designed to target CD20 molecules on B cell surfaces. They observed how the antibodies crosslinked these CD20 molecules on the cells’ membranes, leading to substantial clusters or accumulations of antibodies at the site of interaction.
This binding process has significant consequences. The accumulation of antibodies on the B cell membrane triggers an immune response by activating the complement system—an important aspect of the body’s defense mechanism. The complement system plays a role in promoting inflammation and can ultimately lead to the elimination of the targeted cancer cells by the immune system. The team’s study revealed an intriguing detail: all four of the antibodies under investigation, despite belonging to two distinct categories—type I and type II—crosslinked CD20 molecules in a similar manner. This challenges a long-standing belief that type I and type II therapeutic antibodies function differently. Until now, the common understanding was that type I antibodies promoted immune cell destruction via different mechanisms than type II antibodies. However, this research shows no significant difference in how these antibodies operate, further complicating the established classification system.
The research also uncovered fascinating new insights into the structural changes in the B cells upon antibody binding. Specifically, the antibodies were found to bind most effectively to CD20 molecules on micrometer-long protrusions of the cell membrane, called “microvilli.” These microvilli act as antenna-like structures on the B cell surface. Upon antibody binding, the antibodies cause these protrusions to grow more pronounced and align together, ultimately leading to a polarizing effect in the B cells. This leads to the creation of a unique “hedgehog-like” shape in the B cells, where the protrusions are concentrated on just one side of the cell.
Such changes in B cell shape are not just structural but could have a significant functional role. According to Dr. Arindam Ghosh, the lead author of the study, the new morphology might imply that the B cells are preparing to interact with other immune cells, forming what could be considered an immunological synapse. This synapse could initiate communication between the B cells and other critical immune system components, such as macrophages and natural killer (NK) cells, which are essential for killing cancer cells. If this hypothesis proves correct, it suggests a powerful mechanism by which therapeutic antibodies might not only directly target and destroy B cells but also activate other parts of the immune system to enhance the therapeutic response.
This new understanding has profound implications. The reclassification of therapeutic antibodies based on these findings might lead to more effective treatments, optimizing the immune response against cancer. By confirming the hypothesis that therapeutic antibody-induced polarization leads to interactions with other immune cells, it could lead to novel strategies aimed at boosting the effectiveness of current antibody-based treatments.
The study opens up exciting possibilities for future research, particularly in the areas of cancer immunotherapy and drug design. It will also encourage reexamination of clinical practices and the potential development of more personalized approaches to treatment, wherein antibody therapies might be tailored based on an individual’s specific immune system and cancer type.
Dr. Ghosh and the team from JMU are now focused on further exploring these early observations, trying to validate whether their assumption of antibody-induced immunological synapse formation is correct. This would represent a critical next step in understanding how antibodies may potentiate immune responses, and it could contribute significantly to improving the design and application of immunotherapies for blood cancers and potentially other malignancies as well.
This breakthrough demonstrates the remarkable progress in cancer research and immunotherapy. It highlights the importance of fundamental scientific discoveries in making medical treatments more precise and effective. The introduction of the LLS-TDI-DNA-PAINT method, along with its applications, showcases the power of new technologies in unveiling the molecular-level dynamics of therapeutic antibody action. As this research progresses, it is poised to revolutionize the way scientists and clinicians approach cancer treatment, advancing toward a future where therapies can be optimized for individual patients to achieve the best possible outcomes in the fight against cancer.
Reference: Arindam Ghosh et al, Decoding the molecular interplay of CD20 and therapeutic antibodies with fast volumetric nanoscopy, Science (2025). DOI: 10.1126/science.adq4510