Most materials expand when heated and contract when cooled. This behavior is known as thermal expansion and is typical for most solids. However, there are certain materials that defy this rule, exhibiting negative thermal expansion (NTE), meaning they shrink when exposed to increasing temperatures and expand when they cool down. One such material that has recently garnered attention for its unusual thermal properties is lithium titanium phosphate (LiTi₂(PO₄)₃, often abbreviated as LTP). This material could offer an innovative solution to a significant issue facing lithium-ion batteries: their performance in cold environments.
As portable electronic devices, electric vehicles, and renewable energy storage systems rely increasingly on rechargeable lithium-ion batteries, their efficiency in low-temperature conditions has become a growing concern. In cold climates or at lower temperatures, lithium-ion batteries experience a drastic decline in performance. This issue is especially troubling for industries that depend on reliable energy storage and power delivery, such as electric cars, aerospace, and military applications. As temperatures drop, the performance of these batteries diminishes rapidly due to factors like slowed ion diffusion within the battery’s electrode material.
To address this challenge, scientists have long sought materials and designs that can minimize the performance losses in cold temperatures, but these countermeasures often come at the cost of increased manufacturing complexity or reduced overall efficiency. Some solutions include integrated heaters to warm the battery, special electrode coatings, or using electrolytes with higher conductivity. However, these approaches often add expense and complexity to battery production, limiting their practical benefits. This is where lithium titanium phosphate, a material with negative thermal expansion, comes into play.
A team of researchers from Donghua University, Fudan University in Shanghai, and Inner Mongolia University in Hohhot has proposed a novel solution for improving battery performance in cold environments. Their approach centers on using electrode materials made from electrochemical energy-storage materials that exhibit NTE properties, such as lithium titanium phosphate. Led by researchers Liming Wu, Chunfu Lin, and Renchao Che, the team has published their findings in the journal Angewandte Chemie International Edition, demonstrating the material’s effectiveness for use in rechargeable battery electrodes.
What makes lithium titanium phosphate an intriguing candidate?
Lithium-ion batteries and other rechargeable batteries based on metal ions are indispensable in today’s technological landscape, providing energy for everything from consumer electronics to power vehicles and storing renewable energy generated from solar and wind sources. Under normal conditions, lithium-ion batteries perform well—faster charge and discharge times, and relatively long life cycles—but the performance degradation in cold environments is a well-known limitation.
This performance decline at lower temperatures is largely due to the fact that at lower temperatures, the lithium ions inside the battery move more slowly within the battery’s electrodes. This slowed ion diffusion reduces the efficiency with which energy can be stored or released, negatively affecting the battery’s capacity and performance.
The cold-induced slowdown of lithium ion movement within battery electrodes has been a difficult hurdle to overcome for scientists and engineers. Countermeasures, such as using warmers to heat up the battery or more advanced, temperature-tolerant electrolytes, do help mitigate the issue. However, these solutions either complicate the manufacturing process or lead to an overall drop in efficiency. The ideal solution, then, lies in the development of electrode materials that themselves perform better in cold temperatures—hence the interest in materials like lithium titanium phosphate, which has demonstrated a unique behavior in low-temperature conditions.
How does LTP solve the problem?
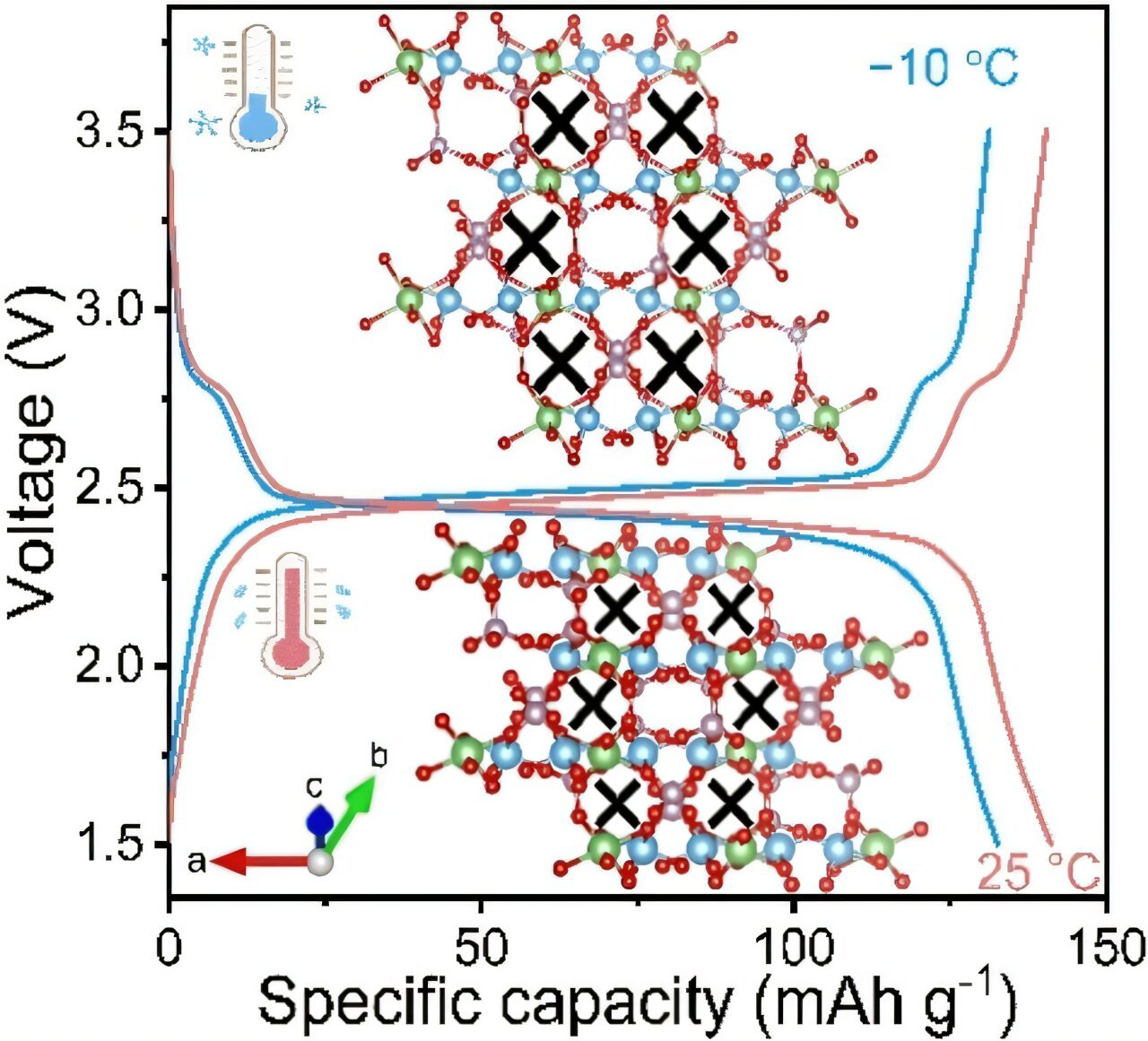
When examined at the molecular level, LTP reveals its unique characteristics. This material has an exceptional ability to expand when it is cooled—opposite of what most materials do. The key to this property lies in its crystal structure, which is composed of a three-dimensional lattice of TiO₆ octahedra and PO₄ tetrahedra. Within this arrangement, the lattice forms open cavities and channels, providing an ideal structure in which lithium ions can settle.
When LTP cools down, its structure undergoes a slight elongation along one of its crystal axes, facilitating a flexible arrangement of atoms that encourages lithium ion movement. At low temperatures, particularly as the material cools, the atomic vibrations in the lattice change, enhancing the ability of the material to store and transport lithium ions. More specifically, the vibrations cause certain oxygen atoms within the lattice to undergo special transverse oscillations, increasing the distance between them. This results in the expansion of the cavities within the lattice, making it easier for lithium ions to navigate and diffuse through the electrode material.
These changes in the crystal lattice at lower temperatures allow the diffusion of lithium ions to remain effective even as temperatures drop. In practical terms, the lithium ions inside the battery can continue to move through the LTP electrode material at a rate that is about 84% of the ion movement seen at a more typical room temperature (25°C), even at −10°C. This behavior represents a significant improvement over conventional battery electrode materials, which often see much greater declines in ion diffusion as temperatures decrease.
The research team conducted extensive spectrometric and electron microscopic analyses of LTP, supported by computer modeling, to better understand these behaviors. The results were promising: electrochemical tests of carbon-coated lithium titanium phosphate at temperatures as low as −10°C showed that the material maintained excellent performance. In particular, it demonstrated a high electrochemical capacity and rapid charge-discharge capabilities, as well as sustained performance over 1,000 charge/discharge cycles.
The material’s ability to maintain high performance at temperatures well below freezing makes it an ideal candidate for use in applications that require dependable battery operation even in cold conditions, such as electric cars operating in winter climates, military field operations, or space exploration missions.
The significance of negative thermal expansion in battery technology
Materials exhibiting negative thermal expansion are exceptionally rare, yet they hold vast potential for a wide range of technological applications. In the context of rechargeable batteries, NTE materials offer a way to effectively mitigate one of the most significant performance challenges faced in cold climates: the slowing of ion diffusion in battery electrodes.
The ability of lithium titanium phosphate to enhance its ion transport properties at low temperatures could make it a game-changer for lithium-ion batteries used in extreme conditions. As industries continue to push for improved energy storage solutions, materials like LTP provide new avenues for advancing battery technology, offering both higher performance in colder temperatures and longer battery lifespans.
While challenges remain in terms of scaling up the use of LTP as an electrode material, these findings are a critical step forward. Researchers are hopeful that through further development and refinement, lithium titanium phosphate and other NTE materials could play a transformative role in the next generation of energy storage solutions, ensuring that lithium-ion batteries can operate efficiently in a broader range of temperatures and environments.
Reference: Qiao Li et al, Negative Thermal Expansion Behavior Enabling Good Electrochemical‐Energy‐Storage Performance at Low Temperatures, Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202419300