Biofilm is an essential yet troublesome phenomenon that can impact both our daily lives and industries across the world. If you’ve ever noticed a fuzzy feeling in your mouth after skipping a brushing, you’ve encountered a biofilm—a slimy, sticky layer of bacteria. Common on teeth, river rocks, and even medical devices, biofilms are more than just an inconvenient coating; they are a significant challenge in medicine, engineering, and other fields. In hospitals, biofilms on catheters, implants, and other medical devices pose serious problems, as they can shield bacteria from antibiotics, making infections far harder to treat. In the industrial sector, biofilms contribute to everything from clogged pipes to equipment corrosion. This growing problem has prompted scientific communities to seek ways to prevent biofilm formation. Now, a breakthrough study from the University of California, Riverside (UCR) could be a game-changer for both healthcare and industry by revealing a naturally occurring chemical found in plants that might hold the key to stopping biofilms before they can form.
The research, led by Katayoon Dehesh, a distinguished professor of molecular biochemistry at UCR, uncovered the role of a particular metabolite known as MEcPP. This small molecule, previously recognized for its importance in plant stress response and metabolism, has now been shown to possess the ability to prevent biofilm formation in bacteria, including notorious strains like E. coli. According to Dehesh, the discovery offers a wide range of possible applications, from improving medical outcomes to reducing industrial corrosion.
To understand the significance of MEcPP, it’s important first to grasp the role of biofilms. Essentially, biofilms are communities of microorganisms—mostly bacteria or fungi—that aggregate on surfaces and are encased in a slimy, protective layer. While biofilms are a natural part of many ecosystems, such as riverbeds and on plant roots, they can cause significant problems when they form in environments where cleanliness and sterility are critical. Plaque on your teeth is a prime example of a biofilm—a mass of bacteria coated in a slimy substance. Biofilms are not limited to personal hygiene or environmental surfaces, however; they form on medical devices like catheters, stents, pacemakers, and implants. These biofilms can make infections extremely difficult to treat, as the bacteria embedded within these slimy structures become resistant to antibiotics. In a clinical setting, this protective barrier shields the bacteria from drugs and immune system responses, often leading to chronic infections.
Beyond healthcare, biofilms pose a significant challenge in industrial applications. In systems reliant on smooth, clean surfaces—such as water pipes, food processing equipment, and industrial machinery—biofilms accumulate and create problems like corrosion, clogging, and contamination. Traditional ways of managing biofilms often rely on chemical treatments that can be harmful to the environment, inefficient in the long term, or ineffective as bacteria adapt. More sustainable and effective methods are clearly needed.
This is where the UCR team’s discovery comes in. In plants, the metabolite MEcPP plays a role in stress signaling—responding to environmental cues when a plant faces challenges, like an excess of oxygen in its cells. In response to stress, the plant accumulates MEcPP, which activates protective mechanisms that help the plant cope with unfavorable conditions. When the UCR team explored MEcPP’s potential influence on bacteria, they made an unexpected and significant finding: MEcPP could hinder the initial stages of biofilm formation in bacteria by disrupting their attachment to surfaces. Specifically, MEcPP interferes with the ability of bacterial cells to adhere to surfaces, a crucial first step in biofilm formation.
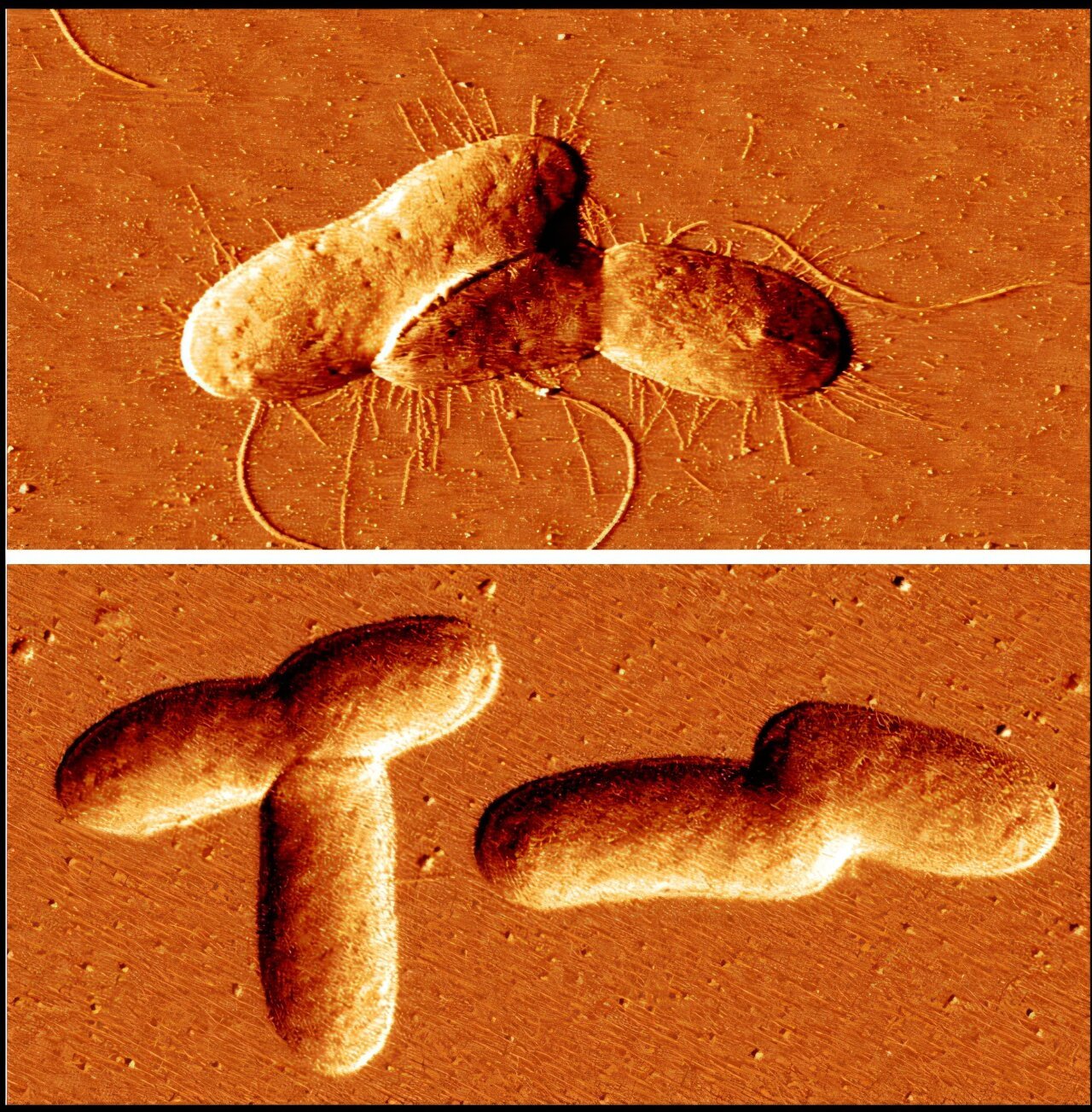
Bacteria rely on structures called fimbriae to anchor themselves to surfaces. These hair-like appendages are the foundation of biofilm formation, enabling bacteria to cling to medical devices, human tissues (like teeth), and industrial surfaces. Once they adhere, the bacteria secrete a matrix of protective slime, which encases them in a fortress-like barrier. This “fortress” makes it difficult for cleaning agents, antibiotics, and the immune system to eradicate the bacteria. Without fimbriae, the bacteria are unable to initiate biofilm formation. What the UCR team discovered is that MEcPP disrupts this process by boosting the expression of a gene called fimE. This gene acts like an “off switch” for fimbriae production. When MEcPP activates fimE, it prevents bacteria from forming fimbriae, disarming their ability to form biofilms.
“We’ve shown that MEcPP has the potential to directly interfere with the first steps in biofilm development,” explained Jingzhe Guo, UCR project scientist and the first author of the study. “By disrupting the attachment phase, MEcPP essentially strips bacteria of their ability to establish these microbial fortresses.”
This breakthrough offers broad implications for fields ranging from medicine to industrial engineering. In medical settings, preventing biofilm formation on devices like catheters and stents could mean fewer hospital-acquired infections and better outcomes for patients. In the food and beverage industry, where biofilms can lead to contamination and spoilage, MEcPP-based treatments could provide safer and more efficient cleaning methods. In industrial processes, particularly in water systems, MEcPP could help prevent clogging and corrosion, extending the lifespan of infrastructure and reducing maintenance costs.
Perhaps even more exciting is the potential for MEcPP-based solutions to replace or reduce reliance on traditional, often toxic chemicals used to combat biofilms. Current biofilm control methods, such as the application of heavy chemicals, come with risks to the environment, human health, and infrastructure. The natural origin of MEcPP provides a more environmentally friendly alternative, which could be safer for both people and the planet.
“Biofilm prevention is a problem with vast applications across numerous industries,” said Guo. “This molecule offers potential in everything from dental care to water systems. The implications are far-reaching.”
The study of MEcPP represents a key example of how fundamental research in plant biology can intersect with other scientific disciplines, particularly microbiology. Discoveries such as these highlight the interdisciplinary nature of scientific progress, where understanding biological processes in one organism—whether a plant or a bacterium—can lead to insights that transform our approach to real-world challenges.
It is remarkable to consider how plants, which have evolved to survive in specific environments, can provide critical solutions to issues affecting human society today. By responding to stress in their environment, plants generate compounds that not only aid in their own survival but may also be applied to improving human technologies and health. This study exemplifies the potential power of plants, whose stress-signaling metabolites could help provide cleaner, safer, and more sustainable methods for controlling biofilm-related problems.
Looking forward, researchers will continue to explore the potential uses of MEcPP in both medical and industrial applications. Given the robustness of its biofilm-preventing properties, scientists may soon begin developing products incorporating this molecule to prevent biofilm formation in a variety of settings. Moreover, this discovery may prompt the search for other natural compounds in plants, microorganisms, or other organisms that have similar potential for combating the growing problem of biofilms.
Reference: Jingzhe Guo et al, An evolutionarily conserved metabolite inhibits biofilm formation in Escherichia coli K-12, Nature Communications (2024). DOI: 10.1038/s41467-024-54501-w