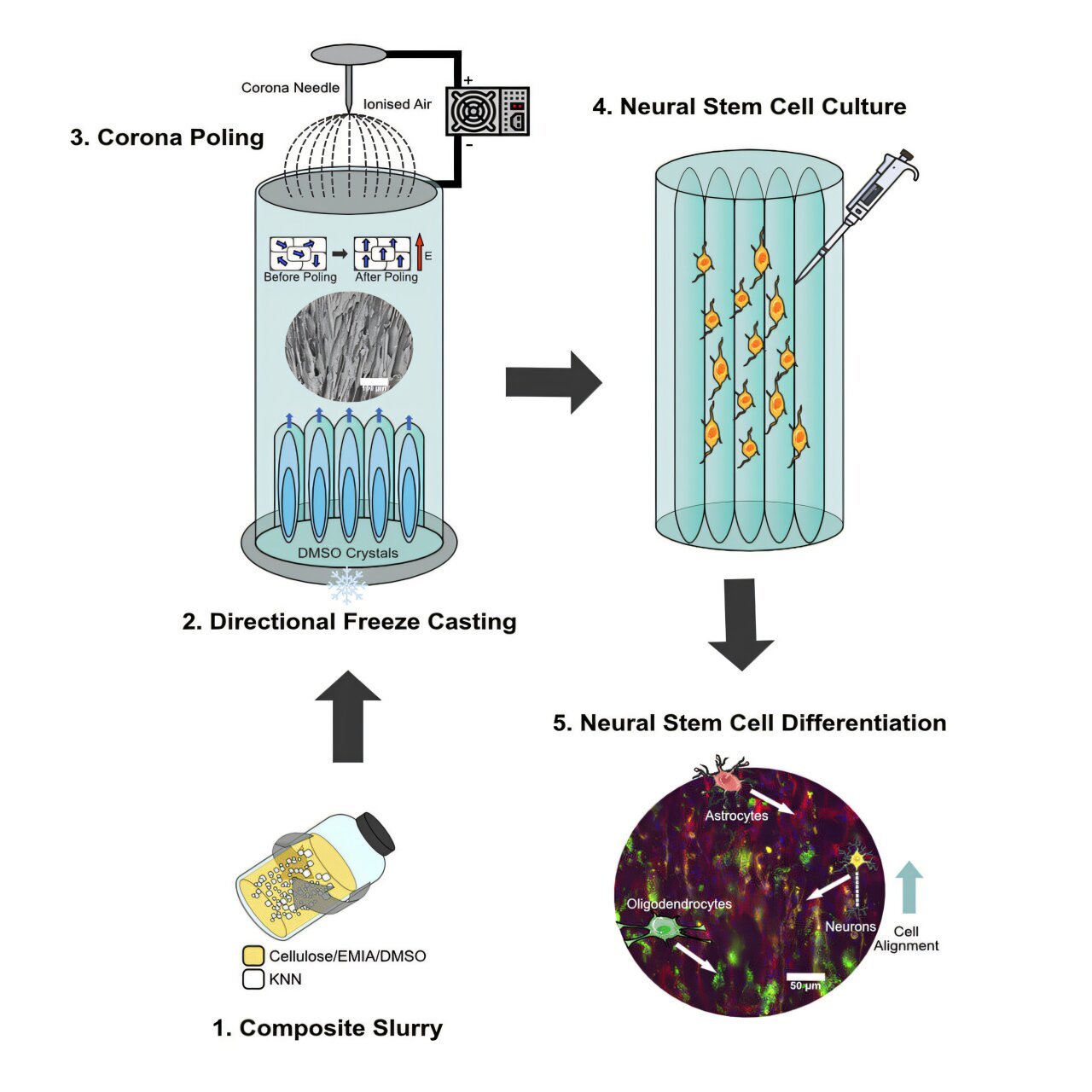
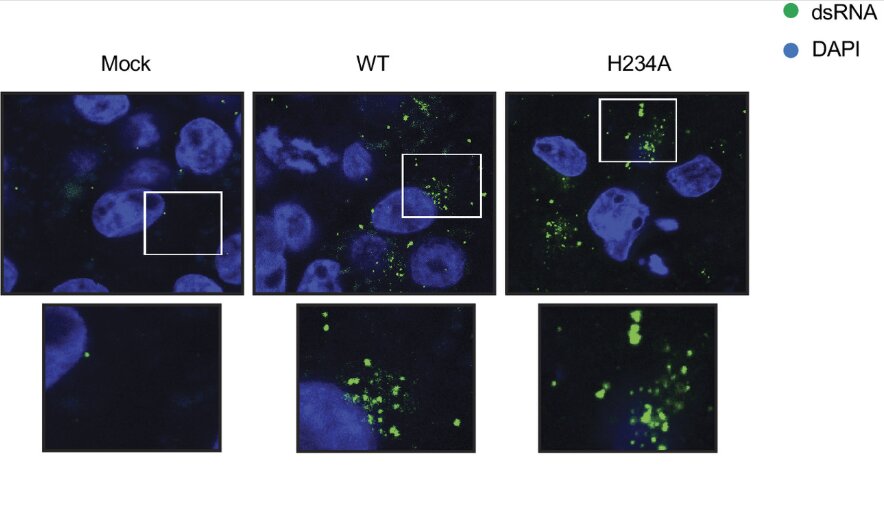
Researchers at the Hospital for Special Surgery (HSS) have made a groundbreaking discovery that could revolutionize the diagnosis and treatment of osteoporosis and other metabolic bone diseases. The study, recently published in JCI Insight, uncovers the pivotal role that circulating osteoclast precursor cells (cOCPs) play in bone loss. This research is the first of its kind to establish a direct connection between these cellular biomarkers and osteoporosis, opening up a promising pathway for earlier detection and more effective therapies for bone diseases.
Osteoporosis is a condition that causes bones to become weak and fragile, leading to an increased risk of fractures. It is particularly prevalent among older adults, with approximately 10 million people aged 50 and older affected in the United States. Unfortunately, osteoporosis is often referred to as a “silent disease” because it typically does not present any symptoms until a fracture occurs. This lack of noticeable symptoms makes it difficult to diagnose early and increases the likelihood of devastating fractures when bones are already severely weakened. According to Kyung Hyun Park-Min, Ph.D., a scientist in the Arthritis and Tissue Degeneration Program at HSS and a lead author of the study, identifying individuals at high risk of osteoporosis before a fracture occurs is crucial to preventing such adverse events.
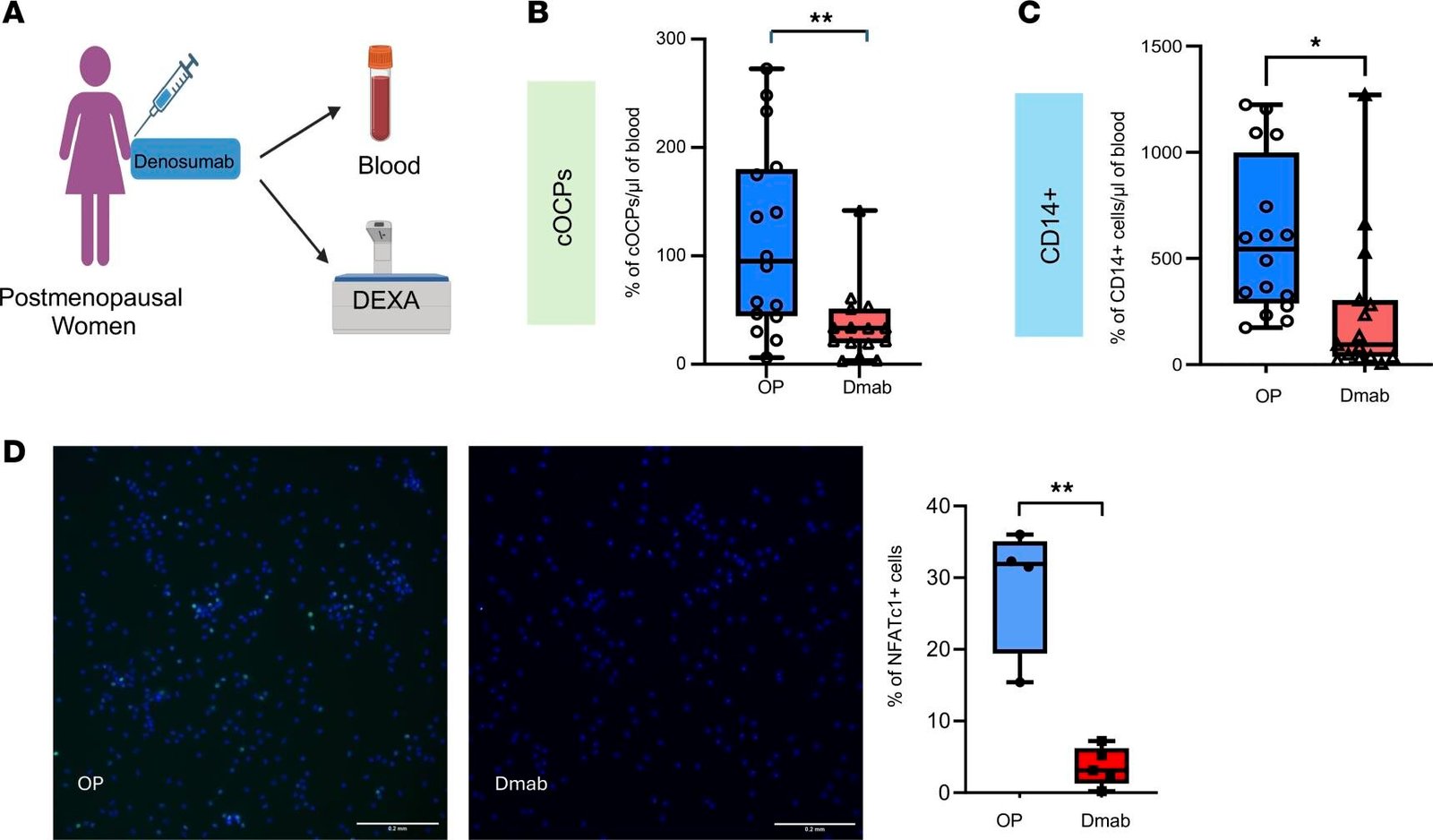
Bone health is maintained through a continuous process called bone remodeling. This process involves two key actions: bone resorption, where osteoclasts break down old bone tissue, and bone formation, where osteoblasts create new bone tissue. Ideally, these processes are balanced, with resorption and formation occurring at equal rates. However, when bone resorption outpaces formation, bone density decreases, leading to weaker bones. The researchers at HSS found that circulating osteoclast precursor cells (cOCPs), which travel through the bloodstream to the bones, play a significant role in increasing bone resorption. These cells fuse with resident osteoclasts in the bones, amplifying the destruction of bone tissue.
Dr. Richard Bockman, MD, Ph.D., an endocrinologist at HSS and a co-author of the study, explained that this research offers a new approach to understanding how bone strength could be maintained by targeting these precursor cells. By affecting the cells responsible for bone damage, it may be possible to develop strategies to slow or halt the progression of osteoporosis and other orthopedic disorders. This new insight also offers a potential target for managing bone health in individuals suffering from metabolic bone diseases.
In their study, Dr. Park-Min and Dr. Bockman collaborated with HSS Endocrinology & Metabolic Bone Service to recruit 44 postmenopausal women. Among them, 16 had untreated osteoporosis, 9 had normal bone density, and 19 had osteopenia, a condition characterized by low bone mass but not yet osteoporosis. Blood tests were conducted to measure the levels of cOCPs in the participants’ blood. The researchers found that women with osteoporosis had significantly higher levels of cOCPs compared to those with normal bone density. This finding underscores the role that cOCPs play in exacerbating bone loss in individuals with osteoporosis, suggesting that these cells could serve as a biomarker for the disease.
While the study sample was small and drawn from a single center, the results have important implications for the screening and treatment of osteoporosis. Currently, the most common method for assessing bone density is the dual-energy x-ray absorptiometry (DEXA) scan. However, this scan is costly, and it is typically only performed once every two years for older populations. The ability to detect elevated cOCP levels through a simple blood test could offer a more cost-effective and timely method for identifying individuals at high risk for osteoporosis, even before they develop the disease. Dr. Park-Min pointed out that identifying people with low bone density who are at risk for osteoporosis could lead to earlier interventions and treatment options that promote bone health.
The potential benefits of this research extend beyond osteoporosis. Circulating cOCPs also play a significant role in joint replacement surgeries. One of the most common complications following joint replacement is aseptic loosening, a condition in which the implant becomes unstable due to bone loss around the implant site. This can lead to the need for additional surgeries and can negatively affect patient outcomes. Dr. Bockman explained that high osteoclast activity is one of the primary factors contributing to this problem, and detecting changes in osteoclast activity before and after surgery could help identify patients at risk for poor outcomes. By monitoring cOCP levels, clinicians may be able to improve surgical outcomes by targeting these cells, potentially reducing complications like aseptic loosening.
In collaboration with the Complex Joint Reconstruction Center at HSS, Dr. Park-Min’s team is conducting additional research to investigate the relationship between cOCPs and the incidence of aseptic loosening following joint replacement surgeries. By detecting elevated levels of these cells in patients prior to surgery, it may be possible to intervene with therapies that target osteoclast precursor cells, potentially improving the long-term success of joint replacements and reducing the need for revision surgeries.
The discovery of the role of cOCPs in bone resorption and their potential as a biomarker for osteoporosis and other bone disorders represents a major step forward in the field of bone health. The ability to identify high-risk individuals earlier, before they suffer fractures or other complications, could lead to more personalized and effective treatment strategies. Furthermore, using blood tests to detect cOCPs would offer a simpler, more cost-effective screening method compared to current techniques like DEXA scans. This could make early detection of bone diseases more accessible to a broader population, ultimately reducing the burden of osteoporosis and other metabolic bone diseases on patients and the healthcare system.
Reference: Kaichi Kaneko et al, Cellular signatures in human blood track bone mineral density in postmenopausal women, JCI Insight (2024). DOI: 10.1172/jci.insight.178977