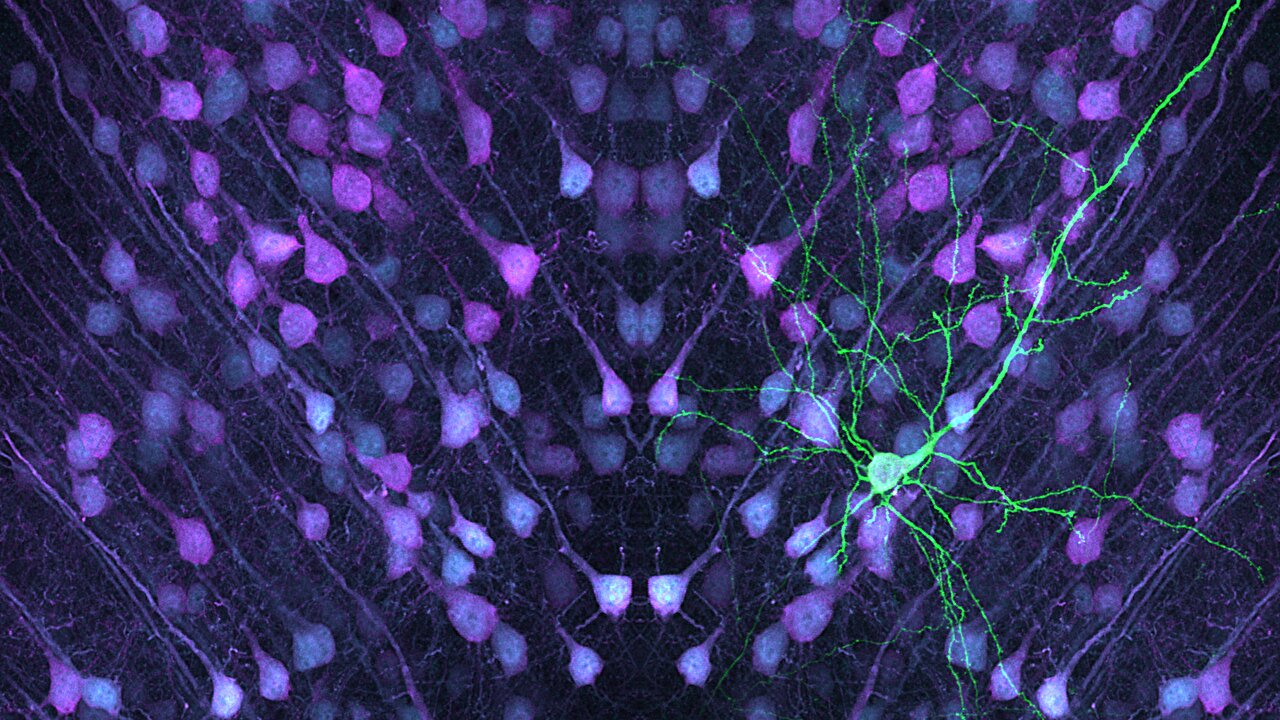
Brain cells known as layer 5 pyramidal neurons play an essential role in the processing and communication of information in the brain. These neurons act as key relay stations in the neural circuits that govern how we think, move, and respond to the world. A recent study led by Professor Joris de Wit from the VIB-KU Leuven Center for Brain and Disease Research has identified critical differences between two distinct types of these neurons, which could explain their varying vulnerability to conditions like autism and schizophrenia. This new research has far-reaching implications in understanding the biological underpinnings of neurodevelopmental disorders.
The findings from Professor de Wit’s team were published in the prestigious journal Nature Communications under the title “Synaptic Signatures and Disease Vulnerabilities of Layer 5 Pyramidal Neurons.” This research focuses on two distinct types of layer 5 pyramidal neurons in the mouse brain: intratelencephalic (IT) neurons and pyramidal tract (PT) neurons. These neurons share a broad anatomical category but perform different functions in relaying and processing brain signals, a fact that the team sought to unravel by studying their unique synaptic features.
Understanding Synaptic Signatures
Layer 5 pyramidal neurons, often described as the brain’s relay hubs, integrate and transmit information to other brain areas. They accomplish this vital task through synapses—tiny junctions where signals are exchanged between neurons via chemical messengers. These connections are not uniform. Rather, different types of neurons utilize different synaptic machinery to receive, process, and distribute these signals to various parts of the brain.
To gain deeper insights into these processes, the research team applied an advanced technique called proteomics, which allows researchers to examine and quantify the diverse proteins present in synapses. Using this approach, they investigated the synaptic profiles of both IT and PT neurons in mice. Proteomics enabled the identification of distinct protein signatures that contribute to the specific ways in which each type of neuron functions. This was crucial, as the proteins found in these synapses directly influence how neurons communicate, process information, and maintain neural circuits.
What the research uncovered was that both IT and PT neurons have some overlapping synaptic proteins but also a number of unique features that set them apart. These unique synaptic proteins could explain why each type of neuron participates in different functions and pathways within the brain. For example, while PT neurons send information directly to distant parts of the brain like the spinal cord, IT neurons are more involved in local brain communication, contributing to processing and integrating complex signals from surrounding regions.
Implications for Disease Vulnerabilities
One of the key breakthroughs in the study was the discovery that these differences between IT and PT neurons may make them differently susceptible to diseases, particularly autism and schizophrenia. According to Professor de Wit, “Mapping the synaptic protein composition of layer 5 neurons is an important step in understanding how these neurons are wired into neural circuits and how this process may be affected by neurodevelopmental disorders.” What sets the IT neurons apart is their higher susceptibility to the onset of autism spectrum disorders. This revelation stems from the fact that the synaptic signatures of IT neurons were found to carry distinct features that may impact their function in developmental brain networks.
Autism, which is often characterized by deficits in social interactions, communication, and repetitive behaviors, has been linked to altered neuronal circuitry, especially in the brain regions involved in communication. IT neurons, with their essential role in the integration and relay of complex brain signals, appear to be particularly vulnerable when there are mutations or abnormalities in synaptic protein composition. This insight provides a potential target for therapeutic interventions aimed at correcting these specific neuronal impairments.
As for schizophrenia, another condition related to disruptions in neural communication, the research also suggests potential differences in the protein profiles of these neurons that could influence vulnerability to the condition. Schizophrenia is associated with issues like disorganized thinking and altered perceptions, many of which stem from disturbed neural communication. The research hints at a possible connection between synaptic proteins and these dysfunctions. Thus, studying how the brain’s circuit wiring is influenced by changes in synapses may ultimately provide more precise insights into what underlies schizophrenia.
These findings are all the more crucial because understanding how these neurons work may enable researchers to identify how specific genetic mutations involved in autism and schizophrenia affect the neurons and how they process information. The study opens avenues for examining how autism-risk genes, for instance, might alter how layer 5 IT neurons are wired, contributing to early developmental disruptions and the emergence of the disorder.
Future Directions
This pioneering work, led by Dr. Gabriele Marcassa and his colleagues, will drive further research in the coming years. Their approach will now expand to mapping the synaptic profiles of other neuron types across different areas of the brain to fully map out how neural circuits function under normal conditions and when altered by disease. With the support of the Simons Foundation, follow-up studies will seek to explore whether autism risk genes specifically affect how IT neurons wire and process information, potentially leading to new insights and targets for therapeutic interventions in autism and similar conditions.
The ability to profile and decode the synaptic protein composition of different neuron types brings with it the possibility of not only diagnosing diseases more accurately but also devising personalized treatments tailored to the neural signatures of specific conditions. Dr. Marcassa emphasizes that this extended approach to mapping synaptic profiles could ultimately lead to a broader understanding of brain health in general. By knowing which neurons are affected and in what ways, treatments may be developed that are more precise, with fewer side effects, offering hope for more effective therapies for neurodevelopmental and mental health conditions.
Moreover, the synaptic profiling of brain cells represents an important step in unraveling one of the most mysterious areas of neuroscience. The connection between brain structure, genetics, and behavior remains one of the biggest challenges in medicine. By gaining a clearer understanding of the synaptic machinery that underpins cognitive function, emotion, and behavior, researchers hope to one day be able to better target interventions for a variety of neurological disorders, ranging from autism to schizophrenia, and even conditions like Alzheimer’s and Parkinson’s diseases.
Reference: Gabriele Marcassa et al, Synaptic signatures and disease vulnerabilities of layer 5 pyramidal neurons, Nature Communications (2025). DOI: 10.1038/s41467-024-55470-w