A groundbreaking advancement in the field of medical research could soon revolutionize the treatment of severe brain and spinal cord injuries, as well as neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Researchers at the University of Bath and Keele University have developed an innovative, electrically active transplantable material that may provide a significant boost to recovery prospects for patients suffering from these life-altering conditions. This 3D piezoelectric cellulose composite, a new type of biocompatible material, can potentially lead to life-changing treatments that promote cell regeneration and recovery for the brain and spinal cord. Published in the esteemed journal Cell Reports Physical Science, the material has been hailed as a major breakthrough in the search for regenerative therapies for central nervous system (CNS) injuries.
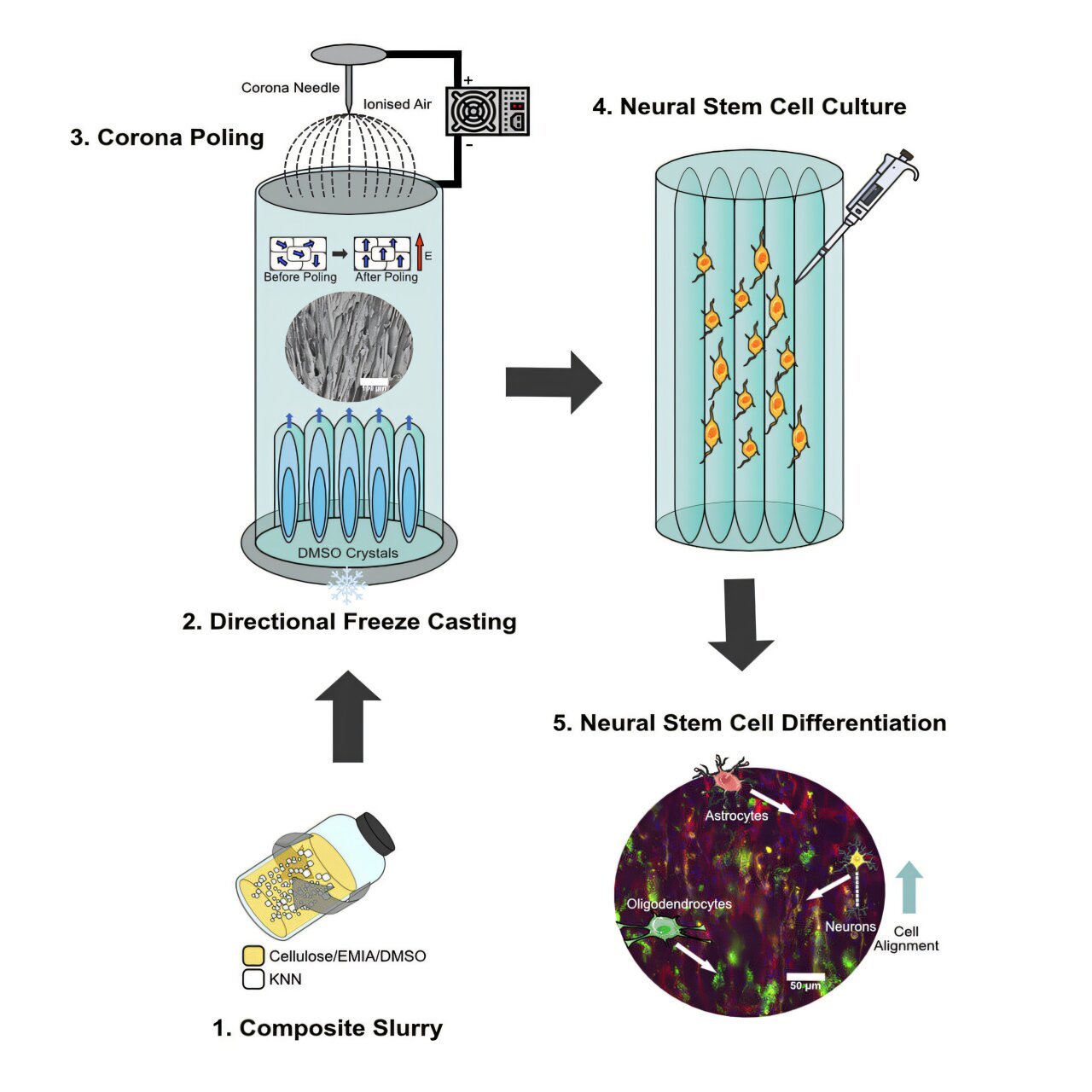
The unique ability of this new composite material to regenerate neural tissue arises from its remarkable multifunctionality. Made of cellulose (a naturally occurring and sustainable substance) combined with potassium sodium niobate (KNN) piezo-ceramic particles, this material serves as a structural scaffold for the precision delivery of neural stem cells (NSCs) into injury sites. The cells then grow and regenerate neurons and other tissues necessary for restoring brain and spinal cord functions. This process could restore motor, sensory, and cognitive functions in individuals whose central nervous systems have been impacted by injury or disease.
CNS injuries have long been a challenge in medicine, with millions of people around the world affected by trauma to the brain or spinal cord. These injuries can result in permanent disabilities and loss of essential functions such as movement and sensation. Currently, effective treatments for CNS injuries are limited, and the ability to regenerate neurons—a process that the body struggles to do on its own after traumatic injury—has been a particularly difficult hurdle to overcome.
However, the development of this new biomaterial marks an exciting new direction in research aimed at overcoming these challenges. The multifunctional piezoelectric composite offers the possibility of developing advanced treatments for CNS injuries that could change the future of medicine. With the expertise of chemists, engineers, and neuroscientists from the University of Bath and Keele University, the research team has taken an important step toward realizing this potential.
Dr. Hamideh Khanbareh, a senior lecturer in the Department of Mechanical Engineering at the University of Bath, emphasized the far-reaching possibilities of this new biomaterial. In her comments, Dr. Khanbareh described the composite as “a groundbreaking biomaterial that has the potential to redefine the prospects of recovery from central nervous system injuries or neurodegenerative diseases.” She noted that this material could bring new hope for patients, offering the promise of regenerating the vital functions that are lost due to injury or disease.
The transformative potential of this composite is rooted in its structure, properties, and the unique combination of components. The composite is created by combining cellulose—an abundant, eco-friendly polymer found in plants and algae—with piezoelectric KNN particles. These piezo-ceramic particles are responsible for the composite’s piezoelectric properties, which play a critical role in stimulating the growth of neural stem cells.
Piezoelectric materials generate an electric charge when they experience mechanical stress, such as when body movements apply pressure or strain on the material. This electrical charge is essential for promoting the growth and differentiation of neural stem cells into mature neural cells, which can then take part in rebuilding damaged tissue in the brain and spinal cord. By applying these piezoelectric cues, the scaffold offers an environment conducive to the effective regeneration of neural tissue, enhancing healing outcomes and improving the chances of a successful recovery for individuals suffering from CNS injuries.
Additionally, the structure of this cellulose-based composite is designed to guide stem cells in specific directions, optimizing the growth and development of cells. This directionality mirrors the natural processes in the spinal cord, where cells and nerves grow and function in ordered patterns. By reproducing these natural mechanisms, the material helps to re-establish the crucial electrical pathways that are often disrupted by spinal cord or brain injury.
Furthermore, the composite is not only effective but also highly sustainable. Cellulose is an abundant natural polymer, making it an eco-friendly component of the material. The scaffold is porous, allowing room for newly grown cells to expand and integrate into the surrounding tissues. This enables effective tissue repair, mimicking the natural three-dimensional cellular structure in the body. Additionally, once the implant has served its purpose, the cellulose is biodegradable and dissolves within the body through enzymatic degradation, reducing the need for additional surgical removal.
In order to achieve customized treatments for individual patients, the material can be fabricated into tailored implants that specifically match the shape and size of the injury site. This is done using a technique called directional freeze casting, which ensures that the cells are aligned optimally along the structure, supporting tissue regeneration at the site of injury.
Dr. Vlad Jarkov, the primary investigator of the research and a Ph.D. researcher in Bath’s Department of Chemistry, explained the clinical potential of the technology and its application for personalized medicine. “One way this could be applied would be to use a CT scan of an injury site to model a very precise 3D implant that could address a patient’s specific needs by accurately bridging the gaps caused by injury to their brain or spinal cord,” he explained.
Dr. Jarkov also described the challenges in advancing treatments for neurodegenerative diseases. “Focusing on finding a way to aid the growth of neural stem cells is very challenging,” he remarked. “They are among the most complex cells in our bodies.” Overcoming this challenge required expertise across a broad range of scientific disciplines, and Dr. Jarkov highlighted the importance of collaboration between engineers, materials scientists, and neuroscientists in bringing this material to life.
Although the material has shown great promise, further development is required before it can be used as a routine clinical treatment. Future research will focus on testing the material’s biocompatibility and efficacy in human applications, optimizing the freeze-casting techniques for manufacturing, and securing regulatory approvals from medical authorities. In addition, extensive studies will be needed to further refine the materials and their ability to integrate with human cells. These trials will be key in ensuring the material can work safely and effectively in patients.
The possible future impact of this technology, however, is undeniable. If these challenges can be overcome, the potential for improving the recovery outcomes of those with CNS injuries or neurodegenerative diseases is vast. With the development of this new biomaterial, scientists have taken an essential step toward restoring essential brain and spinal cord functions, offering real hope for those living with the aftermath of life-altering injuries or the devastating progression of diseases like Alzheimer’s and Parkinson’s.
If successful, these piezoelectric cellulose composite implants could set the stage for a new class of medical treatments—using a personalized, sustainable, and multifunctional material to help patients rebuild and repair their neural tissue. This technology promises to open up a future where patients suffering from brain or spinal cord injuries could not only survive but thrive, with improved quality of life and the possibility of recovery that has long eluded modern medicine.
Reference: Vlad Jarkov et al, 3D piezoelectric cellulose composites as advanced multifunctional implants for neural stem cell transplantation, Cell Reports Physical Science (2025). DOI: 10.1016/j.xcrp.2024.102368