Spinal cord injury (SCI) is one of the most significant medical challenges, with profound consequences for the affected individuals’ quality of life. The injury often leads to permanent physical disability, frequently resulting in varying levels of paralysis, loss of sensation, and impaired organ function. Despite the advanced state of modern medicine, SCI remains a formidable condition to treat due to the central nervous system’s (CNS) inherent limitations in regenerative capacity. Unlike other tissues, the adult spinal cord has a very restricted ability to recover once damaged, making it a complex problem to address therapeutically.
One of the key reasons for the challenges in SCI treatment lies in the unique biology of the spinal cord. During the early developmental stages of the CNS, neural stem cells residing in the spinal cord give rise to diverse types of neural cells that collaborate to form complex circuits, responsible for bodily functions such as motor control, sensation, and autonomic processes. These early-stage stem cells are highly proliferative, capable of producing large numbers of neurons and glial cells to support proper neural function and connectivity.
However, as the spinal cord matures, these progenitor cells lose much of their regenerative potential. The adult spinal cord, consequently, has far fewer neural stem cells capable of self-renewing and differentiating into the required cell types for injury repair. Identifying and harnessing the endogenous stem cells that still exist in adult spinal tissue – cells with the potential for differentiation and repair – could be the key to unlocking therapeutic strategies for repairing spinal cord injury and possibly restoring lost functions.
In a groundbreaking study published in Proceedings of the National Academy of Sciences, researchers at the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences, led by Profs. Dai Jianwu and Zhao Yannan, have begun to unravel the complex mechanisms of spinal cord repair post-injury. Their work represents an important step in understanding spinal regeneration and paves the way for developing novel therapeutic approaches aimed at treating SCI.
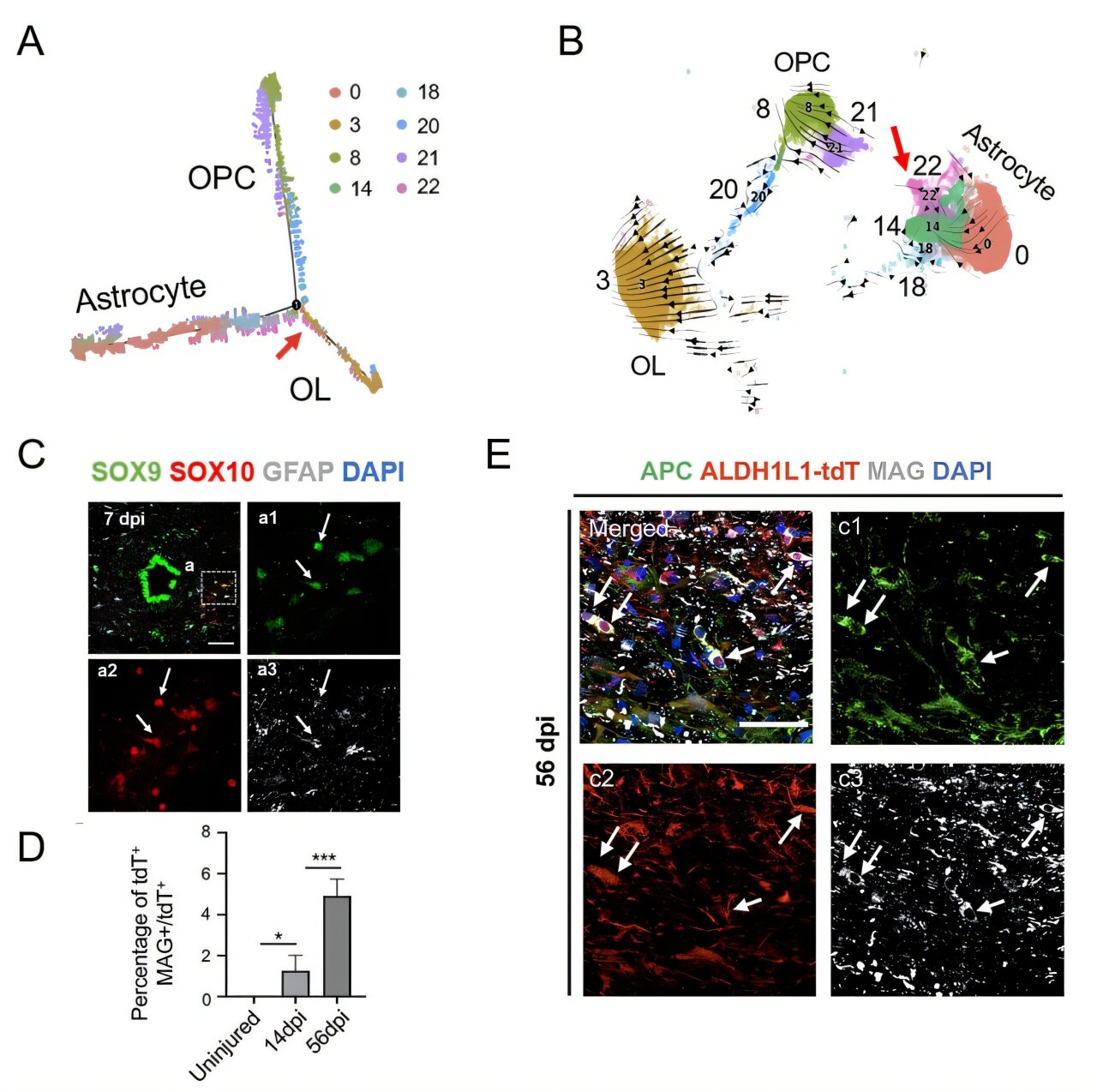
The study focused on the behaviors of specific cell types in the spinal cord, particularly after an injury, utilizing single-cell transcriptomic technology to analyze gene expression at a cellular level. This approach allows for a deeper and more refined understanding of the functional roles and regenerative capacities of different cell populations in the injured spinal cord. One of the highlights of this research was the cross-species comparison between human and rhesus monkey spinal cord development and injury models. This provided an extensive framework to examine how certain cells in different species respond to SCI and to gain insights into the nature of spinal cord repair.
Ependymal cells are one of the most intriguing cell types when it comes to spinal cord regeneration. Ependymal cells line the central canal of the spinal cord and can act as progenitors for new neural cells. During development, these cells have high proliferative and regenerative potential, contributing to the growing structure of the spinal cord. However, as the spinal cord matures, these cells progressively lose their regenerative capacity. This gradual reduction in their ability to differentiate into other cell types makes them a less reliable source for recovery after injury.
The study by Prof. Dai’s team found that ependymal cells are only weakly activated following SCI in adult spinal cords. In both primates and rodents, these cells showed little to no signs of significant proliferation after injury, further substantiating the limitations of these cells in the adult spinal cord. Interestingly, the study noted an essential species difference: in primates, ependymal cells showed even lower levels of reactivity compared to rodents. This finding suggests that the regenerative potential of the spinal cord is inherently more restricted in primates than in rodents, which could have implications for the development of effective SCI treatments across species.
Another critical finding of the study related to astrocytes, a type of glial cell that plays a major role in supporting neurons in the spinal cord and brain. Under normal circumstances, astrocytes do not possess regenerative potential but instead help maintain the homeostasis of the neural environment. Following SCI, however, astrocytes undergo substantial activation, a process known as reactive astrogliosis, in which they proliferate and change their structural and functional properties. Traditionally, reactive astrocytes were viewed as contributing to harmful scarring and inhibition of recovery in SCI; however, recent findings challenge this notion.
In their study, the researchers utilized cutting-edge single-cell and lineage tracing techniques to show that under certain conditions, astrocytes in the injured spinal cord can transdifferentiate into oligodendrocytes—cells that produce myelin, the protective sheath around neurons. This transdifferentiation process has the potential to support remyelination, which is crucial for restoring neural function. Damage to myelin is one of the key pathophysiological features of SCI, so understanding how astrocytes can take on a new identity and contribute to remyelination opens up exciting possibilities for therapeutic intervention.
The study also identified an intermediate population of astrocytes capable of differentiating into oligodendrocyte precursor cells. These cells expressed specific transcription factors, such as SOX10, which were key to driving their transformation into the oligodendrocyte lineage. This provides novel insights into how specific gene regulatory networks in astrocytes can be manipulated to promote myelin repair and facilitate functional recovery in spinal injury models.
Further enhancing this regenerative process, the research team introduced functional material transplantation into the injury microenvironment. The materials used in this intervention were designed to promote a more favorable environment for neural repair by mitigating some of the inhibitory effects of the injury site. This treatment led to a significant boost in the efficiency of astrocyte transdifferentiation into oligodendrocytes, suggesting that modulating the injury site’s microenvironment could be a key strategy in accelerating tissue repair after SCI. Importantly, these findings provide evidence that creating an environment conducive to regeneration may hold more promise for stimulating repair mechanisms than directly trying to reprogram cells.
The results of this study offer powerful insights into spinal cord biology and the regenerative processes that follow SCI. First, it clearly illustrates that the regenerative potential of ependymal cells in the adult primate spinal cord is extremely limited. Second, it provides compelling evidence that astrocytes play an important role in the repair process, showing how they can potentially contribute to remyelination through transdifferentiation. Lastly, the researchers demonstrated how manipulating the injury site microenvironment, via material transplantation, can enhance these cellular repair processes. This research introduces a promising approach in SCI therapy: not only harnessing the endogenous repair capacity of spinal cord cells but also manipulating the local environment to facilitate regeneration.
Given the complexity of SCI and the spinal cord’s limited regenerative capacity, these findings represent an important step forward. By deepening our understanding of cellular behaviors and how they change after injury, we may soon unlock better strategies for inducing repair and ultimately improving outcomes for individuals suffering from spinal cord injuries. While significant challenges remain, advances such as these bring hope that regenerative therapies for SCI could one day become a reality. These findings pave the way for future clinical applications, demonstrating that a combined approach of cell manipulation and microenvironmental modulation holds great promise for transforming the treatment of spinal cord injury in the future.
Reference: Qi Zhang et al, Characterizing progenitor cells in developing and injured spinal cord: Insights from single-nucleus transcriptomics and lineage tracing, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2413140122