Lissencephaly is a rare and severe brain malformation that can disrupt normal neurological function in individuals who suffer from it. The condition is defined by the failure of the brain to develop its characteristic folds, which are essential for normal cognitive and motor function. The malformations that accompany lissencephaly are not just structural but also impact the underlying molecular and cellular mechanisms that govern brain development. Most individuals affected by lissencephaly experience significant neurological issues, such as intellectual disability, seizures, and motor impairments, with no current cure or way to reverse the damage.
A breakthrough Yale study, however, has revealed critical new insights into the mechanisms of lissencephaly and the potential for treatment. Published in Nature on January 1, the study discovered a molecular pathway that could be targeted to prevent and potentially reverse the brain malformations characteristic of some lissencephaly disorders. This research could pave the way for a more effective approach to treating this condition, offering new hope for patients who have traditionally only had symptomatic treatments available to them.
The term lissencephaly comes from the Greek words “lissos,” meaning smooth, and “cephaly,” referring to the head. Typically, the brain’s surface is covered with gyri and sulci, or folds and grooves, that maximize surface area and allow for higher cognitive functions. However, in individuals with lissencephaly, these folds are either incomplete or entirely absent, resulting in a smooth brain appearance. This absence of cortical folds is often associated with developmental delays, seizures, and profound cognitive disabilities. These conditions arise because genes critical for the formation of brain structures are disrupted by genetic mutations. While many genes have been linked to lissencephaly, some patient cases remain genetically undefined, and the underlying biological mechanisms were poorly understood until now.
The Yale study, spearheaded by Angeliki Louvi and Murat Gunel from Yale School of Medicine, set out to explore the underlying biology of lissencephaly in greater detail, utilizing innovative approaches that allowed for the creation of model systems to study the development of affected brains. The research focused on discovering novel genetic contributions to the condition and, most notably, understanding how these genetic mutations disrupt molecular pathways essential for normal brain development.
A significant part of the breakthrough is based on a set of organoids—small, simplified three-dimensional structures that resemble miniature versions of the human brain in development. The study involved growing these organoids using patient-derived cells, reprogramming skin or hair follicle cells from patients into an early state before directing them to develop into neurons. This allowed researchers to analyze how the malformations associated with lissencephaly formed in these organoid models, which accurately mimicked the developmental brain alterations seen in human patients.
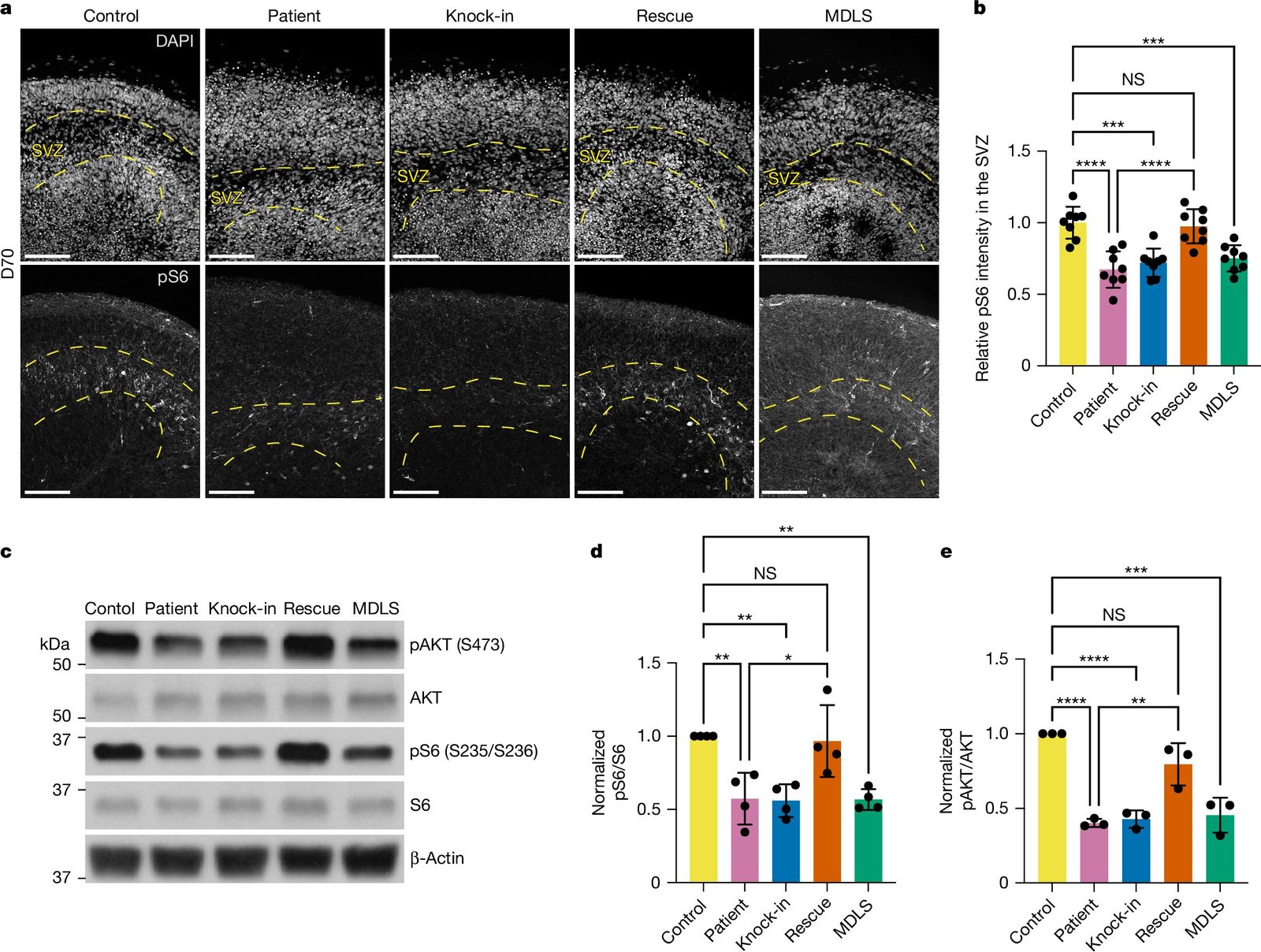
These studies revealed that patients with two different subtypes of lissencephaly had brain organoids that exhibited unusually thick cerebral cortex-like structures. Typically, the cerebral cortex, which is the outer layer of the brain, forms folds during early brain development, but in these affected organoids, the cortex remained abnormally thick and lacked the usual surface folds. This finding closely mirrored the cortical malformations seen in patients diagnosed with lissencephaly.
The breakthrough was not merely a structural observation but also a deep dive into the molecular processes at play in these abnormal organoids. Researchers discovered that there was dysregulation in the mTOR (mechanistic target of rapamycin) pathway. mTOR is a critical protein kinase that regulates various cellular processes, including growth, metabolism, and survival. In many types of disorders, this pathway becomes hyperactive or underactive, disrupting the delicate balance of cellular activity that is essential for healthy development. Previous research had identified that an overactive mTOR pathway contributes to a range of conditions, but this study revealed that in the case of lissencephaly, the mTOR pathway was underactive.
As Louvi and the team observed, when mTOR activity is impaired, the normal processes involved in cell growth and maturation are hindered. In the case of lissencephaly, this meant that neuronal cells did not develop as they should, contributing to the thickening of the cortex observed in these patients. This represents a novel understanding of how a disruption in a key cellular pathway could lead to the brain malformations seen in lissencephaly.
More remarkably, the researchers tested a drug that activates the mTOR pathway, called a mTOR activator. They exposed the lissencephaly organoids to this drug at various stages of development. Remarkably, the introduction of the mTOR activator had a beneficial effect: it not only halted the further thickening of the cortical plate-like region in the organoids but also reversed the abnormal growth in some cases, restoring a more typical brain structure. This discovery represents a crucial first step toward developing a treatment that could potentially alter the course of the disease, rather than merely managing symptoms, such as seizures, which have traditionally been one of the few treatment options available for lissencephaly.
One of the most exciting implications of this research is its potential to provide a treatment that could work across the broader spectrum of lissencephaly disorders, regardless of the specific genetic mutation. The fact that the mTOR pathway was implicated in two genetically distinct types of lissencephaly suggests that targeting this pathway with a therapeutic activator could address various types of the disorder, offering hope for a one-size-fits-all solution. This is particularly significant, as many forms of lissencephaly have been genetically undefined or difficult to treat with current medications.
The ability to prevent or reverse the structural brain malformations characteristic of lissencephaly would represent a major leap forward for neurology, as there are currently no options available that can slow or reverse brain malformations in utero or in the postnatal phase. At present, physicians are limited to attempting to manage the symptoms of lissencephaly, especially seizure management, which can often be difficult to control. Most anti-epileptic drugs do not prove highly effective, further complicating the treatment landscape.
Moving forward, the researchers aim to expand their study to determine if the mTOR pathway is similarly implicated in other types of lissencephaly, including those with undefined genetic causes. They are also focused on further unraveling how and why the underactivation of the mTOR pathway leads to the specific neurodevelopmental defects seen in these brain malformations. Ultimately, these findings suggest that targeted therapies using mTOR activators could form the basis for novel treatments in the future—offering a transformative approach to one of neurology’s most challenging and underexplored disorders.
Reference: Ce Zhang et al, Dysregulation of mTOR signalling is a converging mechanism in lissencephaly, Nature (2025). DOI: 10.1038/s41586-024-08341-9