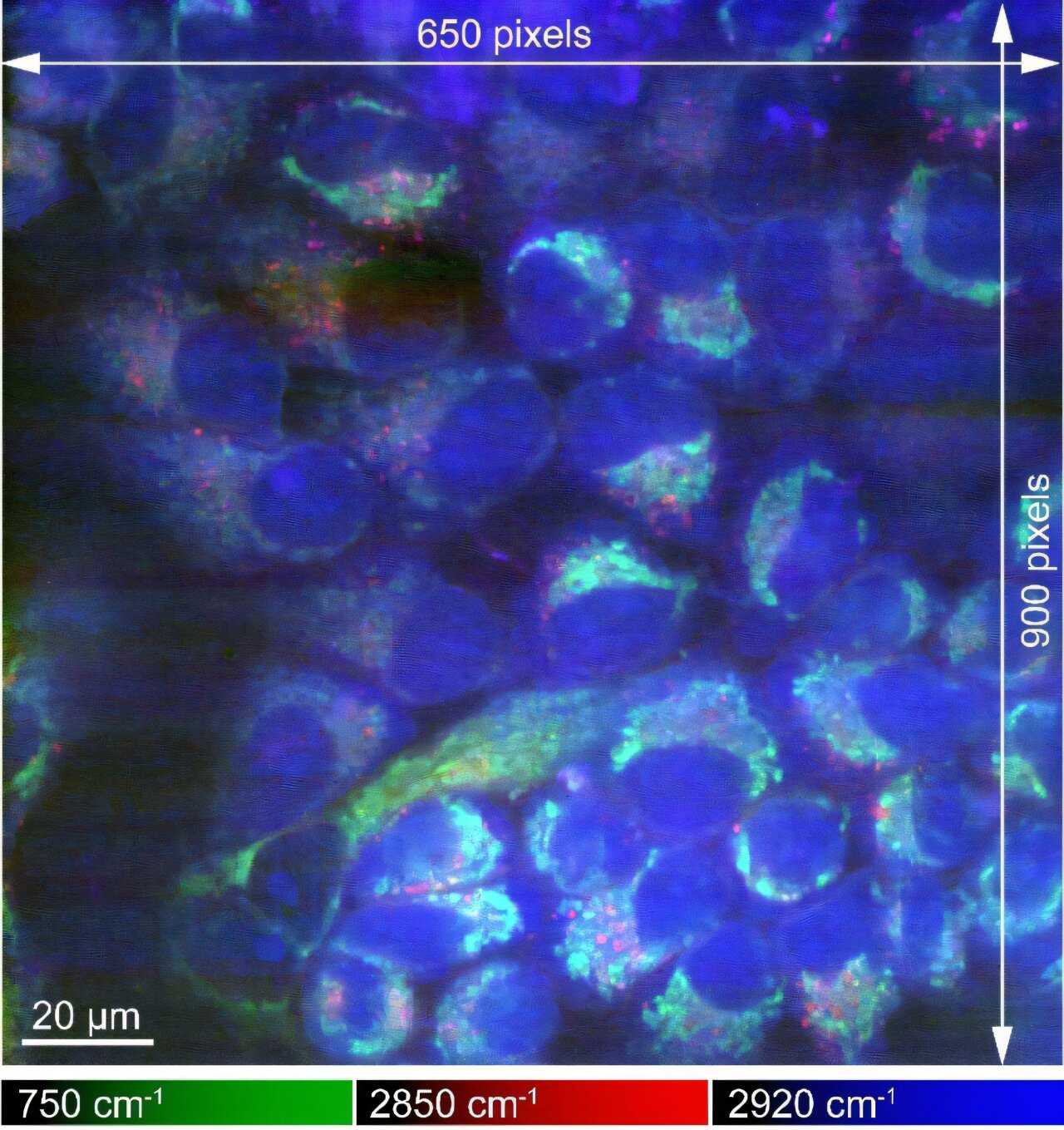
Understanding the molecular and cellular behavior that underpins human biology is a cornerstone of medical advancements. For decades, scientists have strived to improve imaging techniques to capture clearer, more accurate representations of biological processes occurring at the molecular level. One of the most promising developments in this area is the use of Raman microscopy. While traditional microscopy has made it possible to observe cells and tissues, it typically lacks the resolution and specificity needed to analyze the molecular composition of these structures. Raman microscopy, however, has been heralded as a tool that can provide chemical information at the level of individual molecules, enabling a deeper understanding of biological mechanisms. A new study published in Science Advances by researchers from Osaka University has introduced a significant improvement to this technology, enhancing its ability to capture high-resolution images of biological samples in unprecedented detail.
Raman microscopy is based on the principle of Raman scattering, a phenomenon in which light interacts with the vibrational modes of molecules within a sample. When light from a laser hits a biological sample, some of the light is scattered with a change in energy that corresponds to the vibrations of chemical bonds within the molecules. By analyzing this scattered light, scientists can obtain detailed chemical signatures of the molecules present in the sample, such as proteins, lipids, and nucleic acids. This chemical information is invaluable because it allows researchers to study the molecular composition and behavior of biological samples without needing to use chemical dyes or labels, which can sometimes distort the very processes being observed.
Despite its advantages, Raman microscopy faces a significant challenge: the Raman signal is very weak. Biological samples scatter light in a way that can easily be overwhelmed by background noise, making it difficult to capture clear, high-quality images. To overcome this limitation, researchers have focused on enhancing the sensitivity of Raman microscopes, developing techniques to boost the signal strength and reduce noise. The Osaka University team, led by Kenta Mizushima, has made a breakthrough by introducing a novel method that overcomes this challenge while maintaining the integrity of the sample.
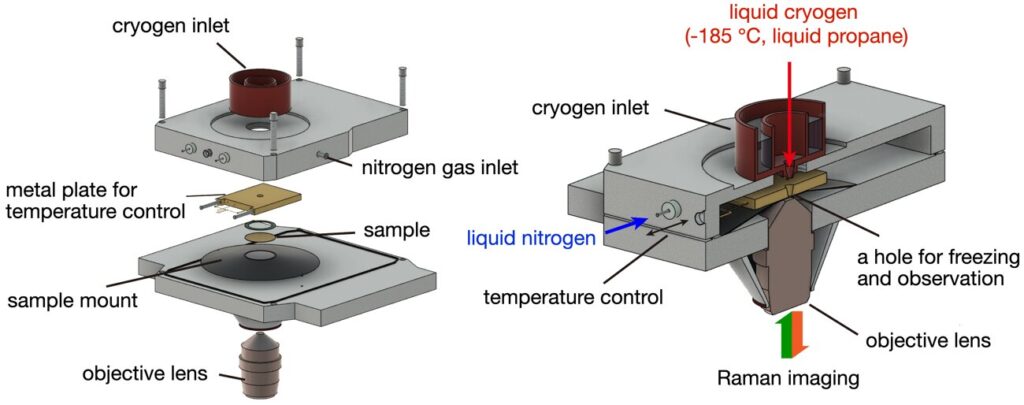
The key innovation in the Osaka University study lies in the ability to image biological samples while they are frozen, or cryofixed, during the Raman imaging process. Freezing the sample at low temperatures prevents the molecules from moving, essentially locking them in place. This step is critical because motion can blur images and reduce the clarity of the resulting data. In traditional Raman microscopy, the movement of molecules within the sample can make it difficult to achieve high-resolution images, as the exposure times needed to capture the faint Raman signal could damage the sample or lead to significant image blur. By freezing the sample, the Osaka University team was able to extend the exposure time without causing any harm to the sample, resulting in brighter, clearer images with much higher resolution.
The technique developed by Mizushima and his colleagues allows them to produce images that are up to eight times brighter than those generated by conventional Raman microscopy. This improvement is a significant leap forward for Raman imaging, making it more suitable for studying complex biological systems where clarity and accuracy are paramount. As Mizushima explains, the motion of the molecules in a sample is one of the main causes of blurry images in Raman microscopy. By freezing the samples, the team was able to eliminate this issue, enabling longer exposure times and better signal-to-noise ratios. The result is a clearer, more detailed view of the biological processes taking place within cells.
In addition to improving image quality, the new cryofixation technique offers several advantages over traditional chemical fixation methods. In conventional biological imaging, chemical fixatives are used to preserve the structure of cells and tissues, but these chemicals can alter the molecular composition of the sample, potentially introducing artifacts or distorting the true biological state. Cryofixation, on the other hand, preserves the sample in a way that is more representative of its natural state. The Osaka University team confirmed that freezing the samples conserved the physicochemical states of proteins and other molecules within the cells, ensuring that the Raman images provided a more accurate representation of the biological processes being studied.
One of the most compelling aspects of the new technique is that it does not require any staining or chemical treatment of the samples. Staining is a common method in biological imaging, where dyes or other chemicals are used to highlight specific structures or molecules. While staining can provide useful information, it can also introduce biases or distortions into the data. The ability to image biological samples without any external chemical intervention is a major advantage, as it allows for a more unbiased and representative view of the biological sample.
Raman microscopy is not just a tool for generating beautiful images; it is a powerful method for obtaining valuable chemical and molecular data. The new technique developed by the Osaka University team enhances the capabilities of Raman microscopy by providing not only high-resolution images of cells but also detailed information about the distribution and chemical states of molecules. This dual capability makes Raman microscopy an invaluable tool for understanding the molecular mechanisms that drive cellular processes, such as protein folding, enzyme activity, and the dynamics of cellular metabolism. As researchers strive to achieve a more complete understanding of the molecular basis of diseases, such as cancer or neurodegenerative disorders, this technique will play a critical role in advancing our knowledge.
Furthermore, this new approach to Raman microscopy has the potential to be combined with other imaging techniques to provide a more comprehensive analysis of biological samples. For example, fluorescence microscopy, which is commonly used to study specific proteins or other biomolecules in living cells, can be paired with Raman microscopy to offer both high spatial resolution and detailed chemical information. By combining multiple imaging techniques, researchers can gain a more complete and nuanced understanding of complex biological systems, which is essential for making advances in areas such as drug discovery and disease diagnosis.
The potential applications of this advanced Raman microscopy technique are vast. In medicine, it could be used to study the molecular mechanisms of disease at an unprecedented level of detail, providing new insights into how diseases develop and how they might be treated. In pharmaceutical research, it could help identify potential drug targets by enabling the study of the molecular interactions between drugs and their targets in cells. In addition, it could play a vital role in the development of personalized medicine by allowing for more accurate analysis of patient samples and better understanding of how individual patients’ molecular profiles influence their response to treatments.
The technique could also have a significant impact on the field of material science. Biological samples and synthetic materials often share similar chemical compositions, and Raman microscopy can be used to study the structure and properties of materials at the molecular level. This capability makes the new technique useful not only for studying biological systems but also for analyzing the chemical and structural properties of materials used in a wide range of industries, from electronics to nanotechnology.
Reference: Kenta Mizushima et al, Raman microscopy of cryofixed biological specimens for high-resolution and high-sensitivity chemical imaging, Science Advances (2024). DOI: 10.1126/sciadv.adn0110