Teeth play an indispensable role in our daily lives, enabling us to chew and break down food while contributing to speech and the structure of our faces. At the heart of their durability is enamel, the hardest and most mineralized substance in the human body. Enamel protects the teeth from the immense forces generated during chewing and shields them from the effects of acids and bacteria. However, unlike other tissues in the body, enamel lacks the ability to regenerate or repair itself once damaged, which makes it vulnerable to wear and tear over time.
As people age, enamel often becomes weaker and more brittle, leading researchers to investigate the changes it undergoes over a lifetime. Understanding these changes is crucial for developing strategies to maintain dental health and potentially prevent tooth decay and damage in the long run. A recent study conducted by scientists at the University of Washington (UW) and the Pacific Northwest National Laboratory (PNNL) has shed light on how the composition of enamel evolves with age, offering insights into the role of fluoride and the structural changes that occur over decades.
The study, published in Communications Materials, focused on analyzing the atomic composition of enamel from two human teeth: one from a 22-year-old individual and another from a 56-year-old. By using advanced imaging techniques, the researchers discovered significant differences in the distribution of fluoride ions between the younger and older samples. Fluoride, a mineral commonly added to toothpaste and drinking water to strengthen teeth and prevent cavities, was found in higher concentrations in the enamel of the older tooth, particularly in specific structural regions. These findings raise intriguing questions about how fluoride interacts with enamel over time and its potential implications for long-term dental health.
Enamel is primarily composed of tightly packed mineral crystals arranged in repetitive structures called prisms or rods. These structures, which are about 10,000 times smaller than the width of a human hair, are what give enamel its strength and resilience. However, this intricate crystalline structure also makes it challenging to study enamel at the atomic level. Previous research on enamel has typically been conducted at much larger scales, limiting scientists’ ability to observe the fine details of its composition.
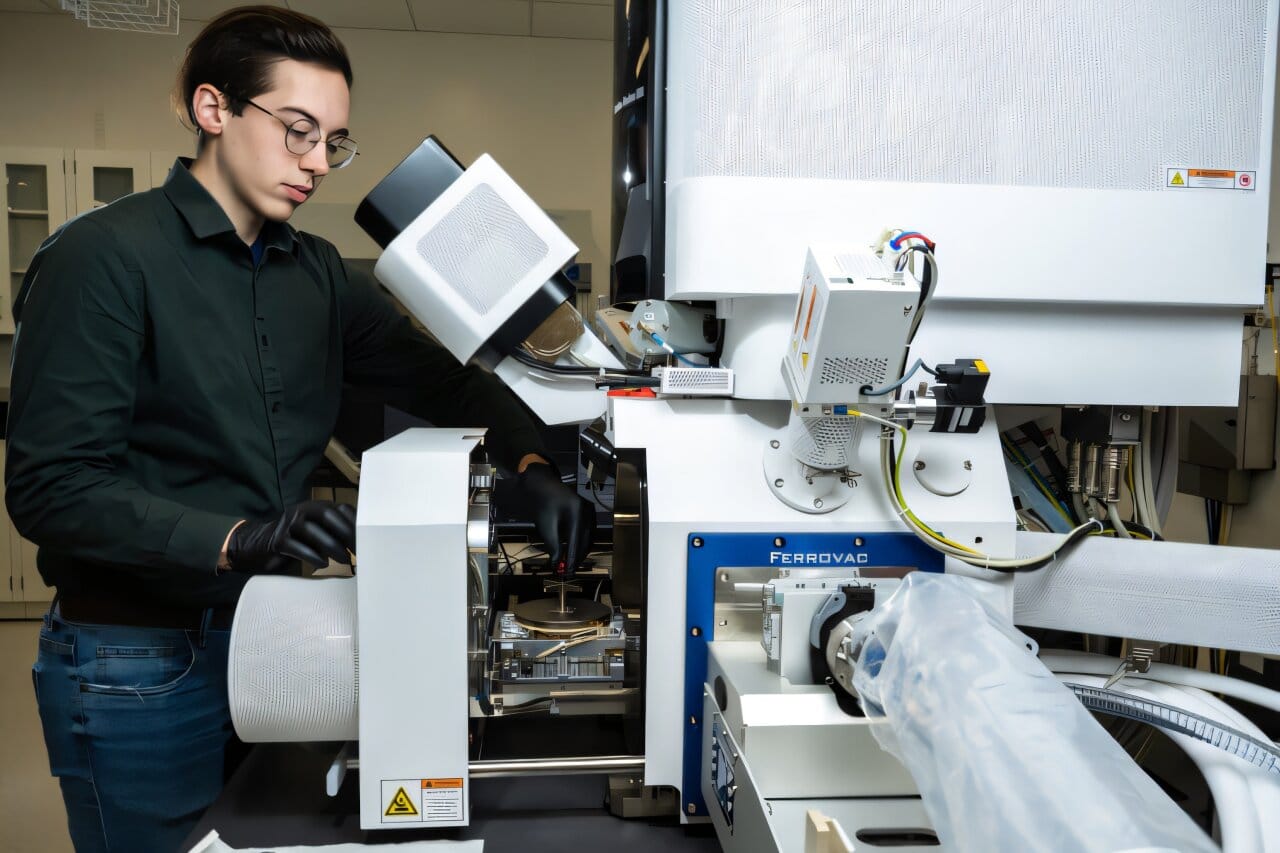
To overcome these limitations, lead author Jack Grimm, a UW doctoral student in materials science and engineering, collaborated with PNNL materials scientist Arun Devaraj to employ a cutting-edge technique known as atom probe tomography (APT). This method allows researchers to create a three-dimensional map of individual atoms within a sample, providing unprecedented insight into the elemental composition and distribution within enamel. The team analyzed multiple samples from each of the two teeth, focusing on different regions of the enamel’s crystalline structure: the core of the prisms, the shell-like coating surrounding the core, and the spaces between the shells.
One of the most striking findings was the elevated fluoride concentration in the enamel from the older tooth, particularly in the shell regions of the prisms. This discovery suggests that fluoride accumulates in specific parts of the enamel over time, potentially contributing to its protective properties. While fluoride is widely recognized for its ability to prevent cavities and strengthen teeth, this study is one of the first to demonstrate how it is incorporated into enamel at the atomic level across a person’s lifespan.
The implications of these findings extend beyond fluoride’s well-known benefits. By understanding how fluoride interacts with enamel at such a detailed scale, researchers can begin to explore new ways to optimize its protective effects. For example, future studies could investigate how factors such as diet, oral hygiene practices, and exposure to acidic foods and beverages influence fluoride uptake and distribution in enamel.
However, the researchers acknowledge that their study is just the beginning. “Of course, the ideal sample would be a tooth from someone who had documented every time they drank fluoridated versus non-fluoridated water, as well as how much acidic food and drink they consumed,” said co-author Cameron Renteria, a postdoctoral researcher at UW. “But that’s not really feasible. So this is a starting point.”
The interdisciplinary nature of the research was key to its success. Devaraj, a metallurgist by training, began studying biomaterials in collaboration with UW professor Dwayne Arola, a co-senior author of the study. Their partnership combined expertise in materials science and dental research, leading to innovative approaches for investigating the complex structure of enamel. “Interdisciplinary science can facilitate innovation,” Devaraj explained, “and hopefully we’ll continue to address really interesting questions surrounding what happens to teeth as we age.”
In addition to fluoride, the researchers are also interested in studying the protein content of enamel and how it changes over time. Enamel contains a small amount of protein, which plays a critical role in its development and structural integrity. The team initially set out to examine whether this protein diminishes with age, but their analysis revealed the prominent role of fluoride distribution instead. Future research could provide more insights into the relationship between enamel’s mineral and organic components and how they contribute to its durability.
As people live longer, understanding the aging process of teeth becomes increasingly important. Enamel brittleness and the development of microcracks are common issues associated with aging, often starting at the outermost surface of the teeth. These changes can lead to more significant dental problems if not addressed. By studying the atomic-scale changes in enamel, researchers hope to develop new methods for preserving its strength and preventing age-related damage.
For now, the message from the study reinforces the importance of fluoride in dental care. “The jury is still out on how aging affects teeth in general,” Arola said. “But the message from dentistry is pretty strong: You should try to utilize fluoride or fluoridated products to be able to fight the potential for tooth decay.”
This groundbreaking research not only highlights the potential of advanced imaging techniques like atom probe tomography to uncover the mysteries of enamel but also underscores the importance of interdisciplinary collaboration in tackling complex scientific questions. As the field of dental materials science continues to evolve, studies like this pave the way for innovative solutions to enhance oral health and improve the quality of life for people of all ages.
Reference: Jack R. Grimm et al, Stratification of fluoride uptake among enamel crystals with age elucidated by atom probe tomography, Communications Materials (2024). DOI: 10.1038/s43246-024-00709-8