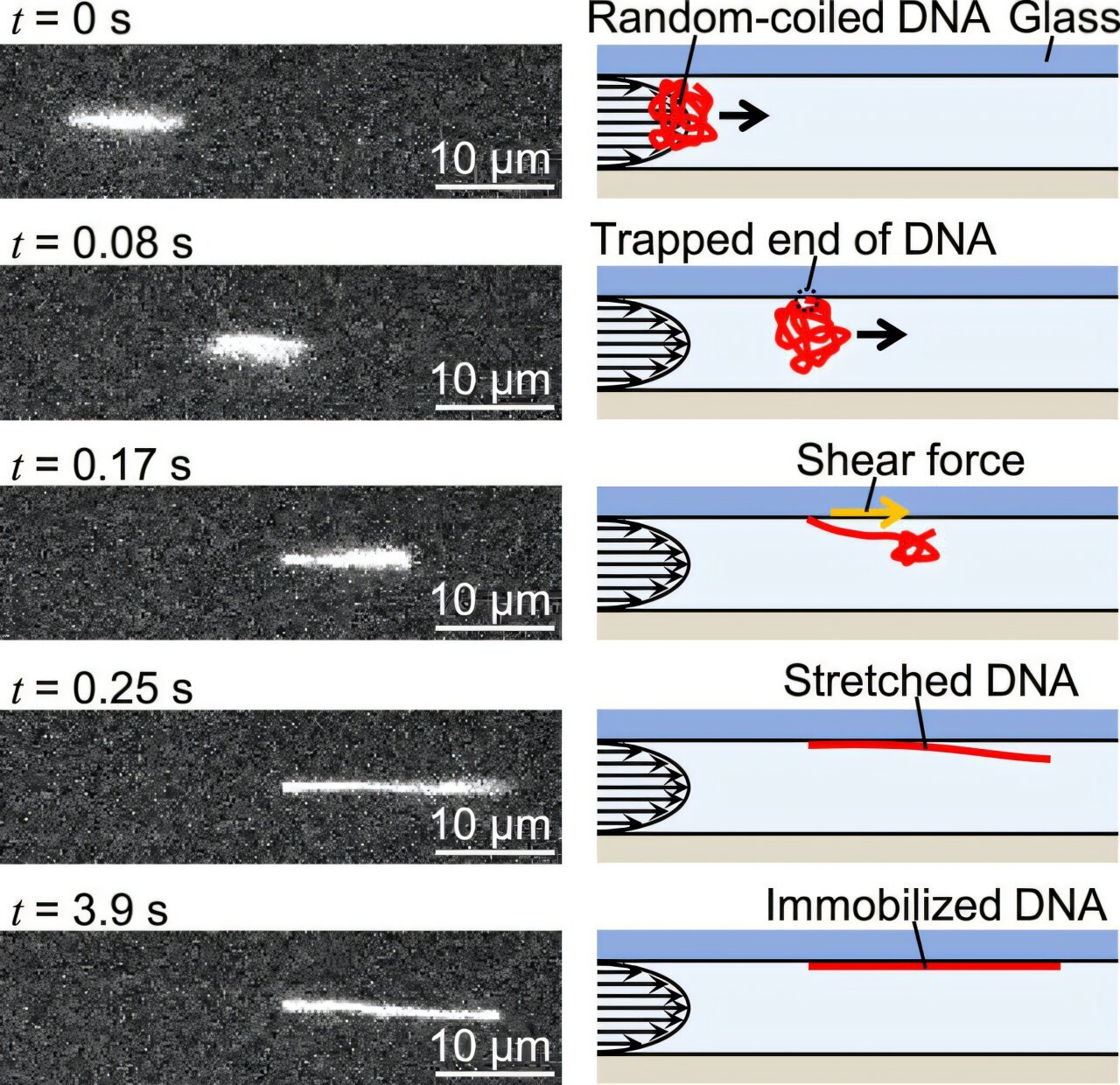
Embryonic development is a process where precise regulation of gene expression is crucial, especially during the transition from oocyte (egg cell) to embryo. This transition, known as the oocyte-to-embryo transition (OET), is a pivotal stage that involves significant changes in the translation of maternal mRNA into proteins. An essential aspect of this regulation is the length of the mRNA poly(A) tail, which is intricately linked to mRNA stability and translational efficiency. The poly(A) tail, a long string of adenine nucleotides added to the 3′ end of mRNA molecules, is central in controlling the fate of mRNA during early development. It can regulate the mRNA’s half-life and its capacity to be translated into proteins.
During early embryonic development, mRNA molecules are predominantly inherited from the mother, and their translation into proteins drives the initial stages of cell division and differentiation. Consequently, ensuring the stability and appropriate translation of these maternal mRNAs is crucial. The regulation of the poly(A) tail has been shown to control this delicate balance of mRNA stability and translation. However, the mechanisms that determine the length of these poly(A) tails in maternal mRNA and how they are maintained during early development have remained poorly understood.
In this context, a new breakthrough study conducted by researchers at the Institute of Biophysics and the Institute of Genetics and Developmental Biology at the Chinese Academy of Sciences has made an important discovery. Published in Nature Communications on January 2, 2025, this collaborative work unveils a key protective mechanism for maternal mRNA poly(A) tails during early embryogenesis.
New Insights into mRNA Poly(A) Tail Protection
The study identified a protein, MARTRE1, which plays a significant role in protecting the poly(A) tail of maternal mRNAs. MARTRE1 acts as an inhibitor to the CCR4-NOT complex, a well-known poly(A) deadenylase complex responsible for shortening mRNA poly(A) tails. This shortening is a process typically associated with the destabilization and degradation of mRNA. The research team found that MARTRE1 binds specifically to maternal mRNAs in early embryos, particularly in cells with characteristics of the 2-cell-like stage (2CLCs), which is a critical early developmental phase.
MARTRE1 was shown to slow down the activity of the CCR4-NOT complex, which in turn leads to a slower rate of poly(A) tail shortening. This slowdown provides two major benefits: it enhances the stability of the mRNA and ensures the mRNA remains available for translation. In the highly dynamic environment of an early embryo, where efficient protein synthesis is necessary for progression, such regulation is crucial for maintaining development.
How MARTRE1 Protects Maternal mRNAs
In vitro experiments, where researchers worked in cellular models, supported the hypothesis that MARTRE1 serves as an important player in the selective translation of maternal mRNA. By acting as a modulator of poly(A) tail length, MARTRE1 helps prevent excessive deadenylation—preserving the integrity of the mRNA and allowing it to be translated into functional proteins during early embryonic development. This protection mechanism is particularly vital at the oocyte-to-embryo transition, a time when the newly forming embryo must rely on maternal mRNAs before its own genome begins to be transcribed and translated.
The researchers also delved deeper into the gene family that MARTRE1 belongs to. Through homology sequence analysis, they identified that MARTRE1 belongs to an under-explored gene family, now called the Martre gene family. This finding opened new avenues for research into similar proteins that could be involved in the regulation of maternal mRNAs and the poly(A) tail dynamics during embryogenesis.
Experimental Evidence from Mouse Models
To validate their findings, the researchers developed a mouse model with a complete knockout of the Martre gene family, allowing them to observe the phenotypic consequences of disrupting this critical regulatory mechanism. These Martre knockout mice showed delays in early embryonic development, providing direct evidence of the importance of MARTRE1 in proper embryogenesis. The delayed development observed in these knockout models strongly indicated that the absence of MARTRE1—leading to the uncontrolled shortening of poly(A) tails—impedes normal development, supporting the notion that controlled poly(A) tail length regulation is essential for early developmental stages.
Furthermore, the researchers employed advanced techniques such as transcriptomics, translatomics, and third-generation sequencing to study the poly(A) tail lengths of mRNAs in both the wild-type and Martre knockout mouse models. Their findings were striking: the presence of MARTRE1 in early embryos ensured that longer poly(A) tails were maintained on maternal mRNAs, thereby enabling more efficient translation. In contrast, the knockout of the Martre gene family led to premature poly(A) tail shortening, resulting in a reduced capacity for protein synthesis from maternal mRNAs.
Implications for Understanding Embryonic Development and Translational Control
This study represents the first identification of an inhibitor of the CCR4-NOT complex during the OET phase in mammals. The protective role of MARTRE1 against deadenylation directly influences the translation of maternal mRNAs in early embryos. Importantly, the regulation of poly(A) tail length appears to be a universal strategy in early embryonic development across species. By safeguarding the stability of long-tailed mRNAs, early embryos can continue to use the maternal mRNA inventory efficiently before the zygotic genome is activated for transcription.
The researchers have presented a compelling new insight into maternal mRNA regulation that could have wide-reaching implications. Understanding how mRNA poly(A) tail lengths are precisely controlled at the start of life opens doors to new therapeutic strategies for managing infertility, early developmental disorders, and potentially even conditions related to the degeneration of mRNA stability.
Broader Implications and Future Research
This study contributes significantly to the emerging understanding of translational control mechanisms during early development, particularly the regulation of maternal mRNA. The discovery of MARTRE1 as a key factor in poly(A) tail protection offers a new molecular target for exploring similar regulatory proteins in other species, which could deepen our insights into early embryonic development. Further research into the Martre gene family and its broader role in gene expression regulation will help clarify the extent to which poly(A) tail regulation shapes development at the molecular level.
Additionally, this finding offers new perspectives on how developmental processes can be modulated by manipulating mRNA stability. As such, the mechanisms unveiled by this study might offer potential strategies for enhancing reproductive technologies, such as improving the quality of in vitro fertilization (IVF) embryos, or for managing diseases related to protein synthesis errors.
Reference: Jing Yang et al, MARTRE family proteins negatively regulate CCR4-NOT activity to protect poly(A) tail length and promote translation of maternal mRNA, Nature Communications (2025). DOI: 10.1038/s41467-024-55610-2
Love this? Share it and help us spark curiosity about science!