Microscopes have been instrumental in the advancement of science, allowing us to study the microscopic world. However, a significant challenge remains in the quest to observe even smaller structures in detail—this limitation is known as the diffraction limit of light. The diffraction limit restricts the resolution of conventional optical microscopes, making it difficult to distinguish fine features below a certain size. Fortunately, super-resolution imaging techniques have emerged, offering a breakthrough by overcoming this constraint and enabling the detailed visualization of biomolecular structures that were once thought to be too small to observe. One area where this advancement is especially valuable is in the study of DNA, where the intricate features of molecules must be resolved to unlock critical biological information.
In particular, research published in AIP Advances by Naoki Azuma and his colleagues at Nagoya University demonstrates an innovative approach that enhances DNA imaging through careful control of thermal fluctuations. By focusing on minimizing these fluctuations and using advanced microfluidic techniques, the team developed methods to stretch and immobilize DNA molecules with remarkable precision. This approach ensures clearer, more accurate imaging of individual DNA strands, which is essential for understanding their structure and function at a molecular level.
The Challenge of the Diffraction Limit in Molecular Imaging
To understand the significance of these advancements, it is important to first recognize the role of super-resolution techniques in overcoming the diffraction limit. In standard optical microscopes, the resolution—the ability to distinguish between two closely spaced objects—is typically constrained by the wavelength of light used for imaging. When scientists attempt to look at very small objects, such as molecules, the light begins to blur and merge together, which means finer details become indistinguishable. This is particularly problematic when studying biomolecules like DNA, where small structural variations are key to understanding biological processes.
Super-resolution imaging methods, which include techniques like STED (stimulated emission depletion) microscopy and single-molecule localization microscopy, bypass this limit by exploiting the behavior of fluorescent molecules at the nanometer scale. These advanced techniques enable researchers to visualize features beyond the diffraction limit of light, revealing the fine details of biomolecules. However, for these techniques to be effective in studying DNA, it’s necessary to minimize factors that contribute to blurring, such as the natural movements that occur due to thermal fluctuations.
The Role of Thermal Fluctuations in DNA Imaging
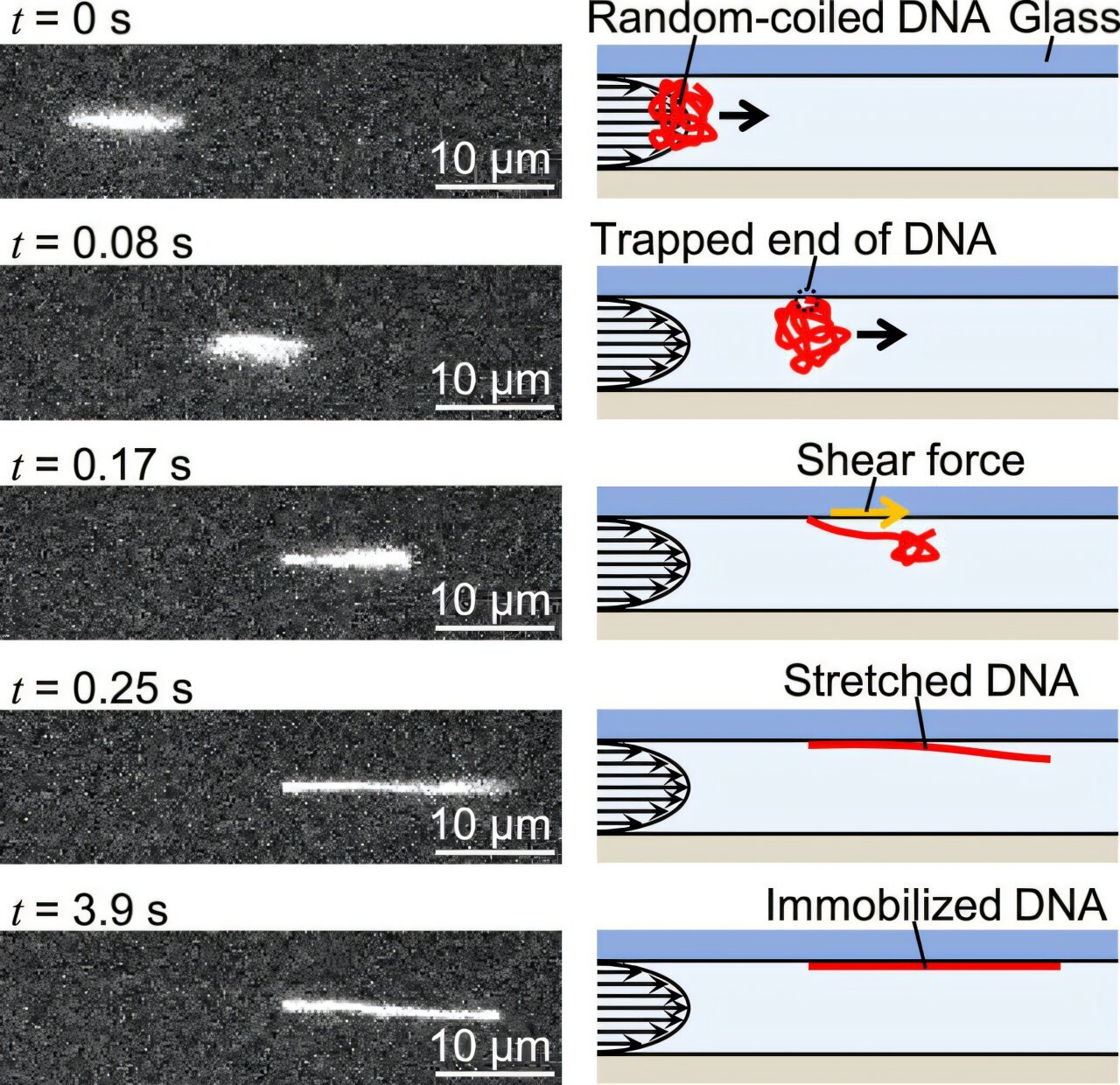
Thermal fluctuations are random, continuous movements of particles caused by thermal energy. These fluctuations occur naturally in all molecules and are especially pronounced at the molecular level. When studying single DNA molecules, these thermal fluctuations can cause the DNA to wiggle or shift, leading to blurry images that hamper the ability to capture fine details. As Naoki Azuma notes, “Immobilizing the molecule essentially ‘glues’ it to a substrate, preventing any movement caused by thermal fluctuations.” This reduction in movement is critical for capturing clear images in super-resolution techniques.
Despite the importance of immobilizing molecules for accurate imaging, researchers have faced challenges in achieving this with DNA. In previous approaches, one end of a DNA molecule was attached to a surface or substrate, while the other end was pulled to stretch the DNA straight. While this did indeed stretch the DNA, thermal fluctuations still induced minor movements that led to blurring. This made it difficult to clearly visualize the structure of the DNA, which must be examined in great detail for studies involving genetic sequencing, molecular interactions, or structural analysis.
Stretching DNA with Precision
In their research, Azuma and his team at Nagoya University set out to solve these challenges by developing a technique that would stretch DNA molecules with precision while minimizing the effect of thermal motion. Their solution involved applying a controlled flow of liquid through a microfluidic channel. The liquid flow creates a shear force that gently uncoils the DNA, causing it to stretch from its natural coiled state into a straight line. This stretching process, called DNA elongation, is crucial for studying the sequence and structure of individual DNA strands.
Controlling the velocity of the liquid flow proved to be a key factor in the precision of the DNA stretching process. By adjusting the flow rate, the researchers were able to fine-tune the amount of shear force applied to the DNA, allowing them to control the degree of stretching—what is referred to as the stretch ratio. This level of control is important for ensuring that the DNA does not stretch too much, which could result in distorted or unusable images, or too little, which would prevent accurate measurements and analysis.
Immobilization and Chemical Bonding
While controlling the flow velocity and shear force helped achieve the desired DNA stretch, Azuma’s team took another step to ensure that the DNA would remain stable during imaging. To prevent any unwanted movements caused by thermal fluctuations or other forces, the researchers utilized a specialized chemical that forms strong covalent bonds between the DNA and a glass substrate. This process essentially “glues” the DNA in place, holding it firmly to the surface during imaging sessions.
This immobilization is critical because it significantly reduces the influence of thermal fluctuations, which otherwise could blur the images taken with super-resolution techniques. In traditional approaches, even slight movement of the molecule can diminish the quality of the image. Immobilizing the DNA helps the researchers capture much clearer, more stable images over short periods of time—often just seconds or minutes.
Applications and Future Directions
The ability to stretch and immobilize DNA with minimal thermal fluctuation has far-reaching implications in molecular biology and genetics. DNA molecules can now be observed with unprecedented precision, allowing scientists to study the specific base sequences, molecular interactions, and structural features of DNA strands. Researchers could examine how proteins interact with DNA, how genes are expressed, or how DNA changes over time with environmental influences—all without the blurring caused by thermal motion.
While the advancements made by Azuma and his colleagues are promising, there are still some limitations in directly visualizing individual base pairs of DNA. However, this work represents a major leap forward in the ability to study DNA molecules and other biomolecules with precision. Azuma’s team envisions future improvements to their methods, focusing on refining both the stretching and immobilizing processes to achieve even higher fidelity in molecular imaging. The ultimate goal is to allow the detailed observation of DNA’s structure and function with such precision that it could revolutionize the fields of molecular diagnostics, personalized medicine, and gene therapy.
By combining advanced super-resolution imaging techniques with careful DNA manipulation and stabilization, the research opens up new possibilities in the study of genetic materials, providing greater clarity than ever before. The work undertaken at Nagoya University is an important contribution to the growing field of molecular imaging and could pave the way for more detailed and accurate investigations into the microscopic world of molecular biology.
Reference: Naoki Azuma et al, Stretching and immobilizing a single DNA molecule on a glass surface using pressure flow in a microchannel for super-resolution imaging, AIP Advances (2025). DOI: 10.1063/5.0223375