Cement production is a cornerstone of modern construction and urbanization, making it one of the most universally essential materials in the world. However, it also stands as the second-largest industrial contributor to global greenhouse gas (GHG) emissions. In fact, the cement industry is responsible for approximately 8% of global CO2 emissions, primarily due to the energy-intensive manufacturing process and the chemical reactions that occur during production. A new study from researchers at the University of Michigan proposes a transformative solution to address this environmental issue by significantly reducing the carbon footprint of cement production without changing the manufacturing process.
The groundbreaking research, which was recently published in the prestigious journal Energy & Environmental Science, introduces a low-cost, scalable approach that could neutralize the most carbon-heavy step in cement production: the creation of calcium carbonate, a key ingredient. If fully implemented, the strategy could dramatically cut down CO2 emissions in cement production, providing an effective pathway to addressing a pressing climate challenge.
Traditional Cement Production and its Environmental Impact
Cement is the most widely used commodity in the world after water. It forms the basis for concrete, which is essential for building infrastructure like buildings, roads, and bridges. To meet the growing global demand—estimated to rise by 50% as urbanization continues—global cement production is projected to increase. The production process for ordinary Portland cement, which is the most commonly used form of cement, has been largely unchanged for over a century.
The traditional manufacturing process involves heating limestone (which is mostly composed of calcium carbonate, or CaCO3) in a rotary kiln at high temperatures to produce calcium oxide (CaO), a critical component in cement. However, this process generates a significant amount of CO2 emissions in two ways. First, fossil fuels used to heat the kilns emit large amounts of CO2, contributing to approximately 40% of the emissions from the process. Second, as the limestone breaks down during heating, CO2 is released due to the chemical decomposition of calcium carbonate. This reaction alone accounts for an additional 60% of the overall emissions.
The cumulative result of these emissions is substantial. With the current global demand for cement on the rise, cement production is expected to be a major driver of CO2 emissions if left unaddressed. Thus, finding an effective method to lower the carbon impact of cement production is an urgent need for both the construction industry and the broader goal of achieving global climate targets.
A Novel Solution: Electrochemical Calcium Carbonate Production
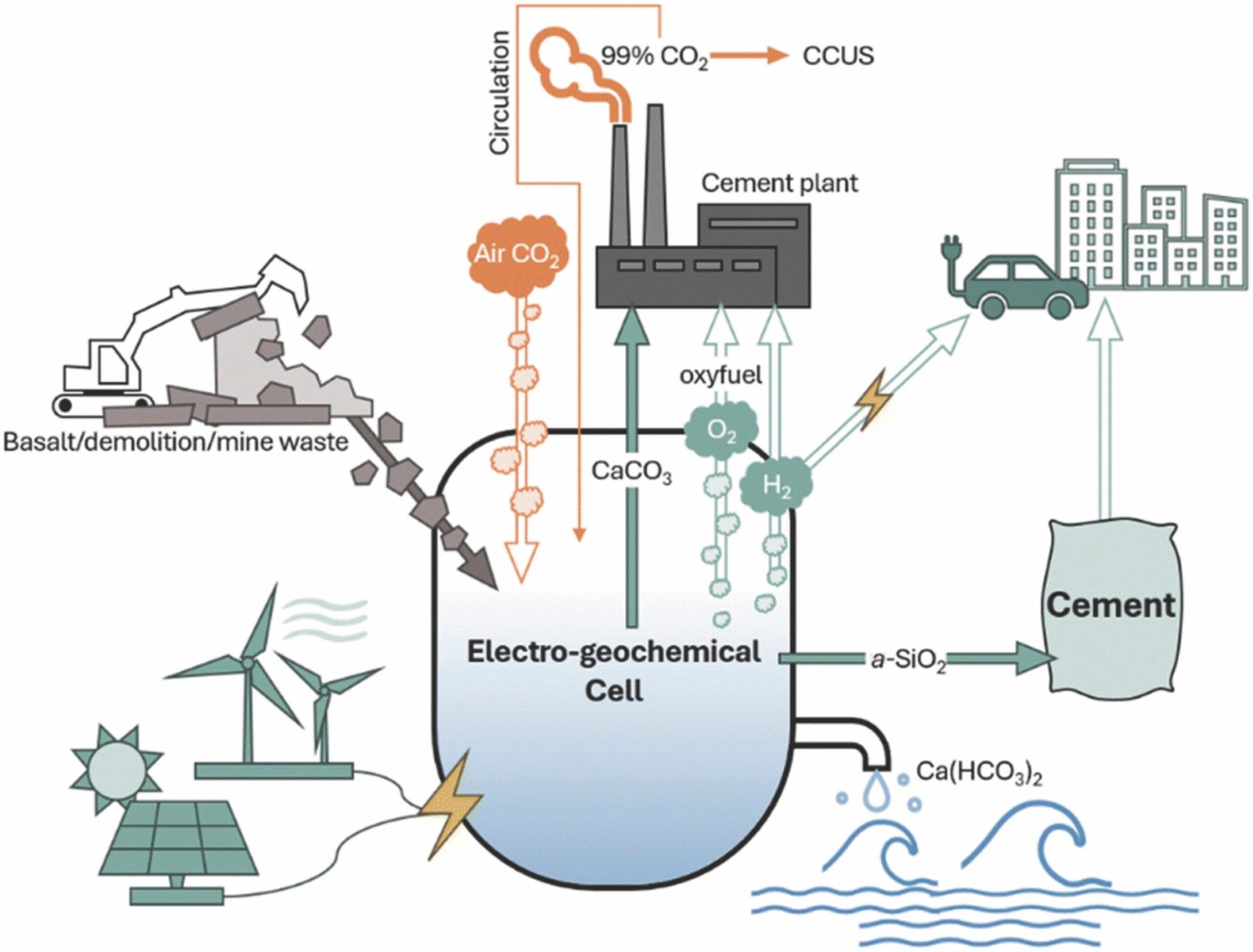
The research team, led by Jiaqi Li, an assistant professor at the University of Michigan’s Department of Civil and Environmental Engineering and a staff scientist at the Lawrence Livermore National Laboratory, has developed a groundbreaking solution that could drastically reduce CO2 emissions during cement production. Instead of relying on conventional limestone, which is the primary source of calcium carbonate, the researchers have developed a way to produce calcium carbonate through an electrochemical process that captures CO2 from the air and binds it with abundant minerals or even recycled concrete.
This new approach uses a process called electrochemical material manufacturing, in which an electrolyzer—a device with positive and negative electrodes—applies an electric potential to water containing a neutral electrolyte. By applying this electrical potential, water at the anode (positive electrode) splits to form oxygen gas (O2) and positively charged protons (H+), while water at the cathode (negative electrode) produces hydrogen gas (H2) along with negatively charged hydroxide ions (OH-). This process creates an acidic electrolyte at the anode and an alkaline electrolyte at the cathode.
At the same time, the protons created in the electrolyzer interact with the calcium silicate, which is the mineral form of calcium found in minerals such as clay or recycled concrete. The protons break down calcium silicate into its constituent parts—solid silica (SiO2) and calcium ions (Ca2+). The calcium ions then react with carbon dioxide (CO2) from the air and hydroxide ions (OH-) to form solid calcium carbonate (CaCO3), which can be used directly in cement production.
Neutralizing CO2 Emissions During Cement Production
What sets this electrochemical process apart is its ability to reduce CO2 emissions at the very step of cement production that currently produces the most carbon. Traditionally, during the heating process, CO2 is released from the limestone as it breaks down. In contrast, this new process captures CO2 directly from the atmosphere and neutralizes the emissions, transforming them into solid calcium carbonate that can be reused in cement kilns.
The end result is a carbon-negative product because the CO2 captured from the air during the electrochemical process offsets the CO2 released from the kiln during production. This novel approach doesn’t just help reduce emissions but provides a closed-loop, circular carbon process in cement manufacturing.
According to Jiaqi Li, the new process “opens a new area in cement production and waste upcycling at scale.” The team’s development could be a game-changer for the cement industry, significantly lowering the carbon emissions traditionally associated with cement manufacturing without requiring an overhaul of existing manufacturing facilities.
Potential for Global Impact
The environmental potential of this method is enormous. Researchers estimate that if this electrochemical approach were implemented globally at full capacity, it could reduce CO2 emissions from cement production by at least three gigatons annually. For context, the global total energy-related CO2 emissions in 2023 reached approximately 37.4 gigatons, and the cement industry’s share of that figure currently stands at 8%. By reducing cement’s contribution to global emissions from 8% to as low as 3%, or potentially achieving net-zero emissions with further integration of carbon capture technologies, this approach could play a transformative role in reducing global carbon emissions.
Moreover, this reduction in CO2 emissions would not just help mitigate climate change—it could also enable the cement industry to become a positive contributor to carbon management. As Wenxin Zhang, a doctoral student at the California Institute of Technology and one of the contributing authors of the study, explains, “The strategy can change the cement industry from a gigaton CO2 emitter to a gigaton-scale enabler for clean energy and carbon management technologies.”
Added Benefits Beyond Carbon Reduction
In addition to producing carbon-neutral calcium carbonate for cement kilns, the electrochemical process also produces solid silica (SiO2), a material that could be used to improve the strength and durability of concrete and mortar. The versatility of the output products enhances the value of the new process by introducing a supplementary material that could make cement and concrete even more useful and efficient for construction.
Furthermore, the electrolyzer produces hydrogen gas, which can be used as a clean, green fuel in various energy applications. Hydrogen has emerged as a potential key player in decarbonizing various sectors, and harnessing hydrogen production as part of this approach represents an additional benefit.
Additionally, the research team has explored the feasibility of using the gas byproducts—especially oxygen and hydrogen—further to enhance the overall sustainability of the process. The hydrogen gas can serve as a green fuel source, while the oxygen and oxy-fuel generated during the electrolytic process could help with carbon capture and storage from flue gas, further enhancing emissions reductions across the industrial ecosystem.
Economic Viability and Scalability
A crucial aspect of any new technological solution is its economic feasibility. For this approach to make a widespread impact, it must be both cost-effective and easily scalable. The research team took the time to assess whether the electrochemical process is economically viable when accounting for potential carbon credit savings. The results were promising. The electrochemical approach proved to be lower-cost and more efficient than existing methods, making it a viable solution that could be readily adopted by the global cement industry.
What’s particularly compelling about this method is that it could be implemented in existing cement plants with minimal or no modifications. As noted by Xiao Kun Lu, a doctoral student at Northwestern University and the lead author of the study, “The present strategy requires minimal or no modification to business-as-usual cement plants, so it has low entry barriers for large cement businesses.” This represents a critical advantage, as the cement industry is vast and deeply entrenched in existing practices. The ability to seamlessly integrate this carbon-capturing process into existing infrastructure could accelerate adoption and lead to immediate benefits.
Collaborative Research Effort
This study was the product of a collaborative effort between some of the most prestigious institutions in the world. The University of Michigan, Lawrence Livermore National Laboratory, Northwestern University, and the California Institute of Technology all contributed to the development of this innovative electrochemical process. By bringing together experts in civil and environmental engineering, chemical engineering, and materials science, the research team was able to create a solution that stands at the forefront of addressing one of the most pressing environmental challenges facing humanity.
Conclusion
Cement production represents a significant challenge in the fight against climate change, but the University of Michigan’s innovative new electrochemical approach presents a solution that could revolutionize the industry. By capturing CO2 from the air and using it to produce calcium carbonate for cement manufacturing, this method has the potential to substantially reduce CO2 emissions, helping to mitigate the environmental impact of one of the world’s most indispensable materials.
As the world grapples with rising CO2 levels and seeks pathways to achieve a net-zero future, this breakthrough offers an exciting opportunity to reshape an entire industry and contribute meaningfully to global sustainability efforts. With its potential for scalability, economic viability, and minimal disruption to existing infrastructure, this electrochemical approach may play a pivotal role in building a greener and more sustainable future for generations to come.
Reference: Xiao Kun Lu et al, Scalable electrified cementitious materials production and recycling, Energy & Environmental Science (2024). DOI: 10.1039/D4EE03529A