Human Immunodeficiency Virus type 1 (HIV-1) targets the immune system, ultimately compromising the body’s ability to fight off infections and diseases. As it infiltrates human cells, it inserts its genetic material into the host’s genome and hijacks the cell’s machinery to replicate. A key aspect of this process involves the virus’s ability to efficiently deliver its genetic payload into the host’s nucleus, a critical step in the infection cycle. Recent research by Martin Beck, Gerhard Hummer, and Hans-Georg Kräusslich, alongside their teams from the Max Planck Institute of Biophysics and Heidelberg University Hospital, provides new insights into the mechanics of this process. Their findings, published in the journal Cell, offer a detailed look at how HIV-1 capsids penetrate the nuclear membrane to integrate viral DNA into the host’s genome.
The HIV-1 Capsid: Structure and Function
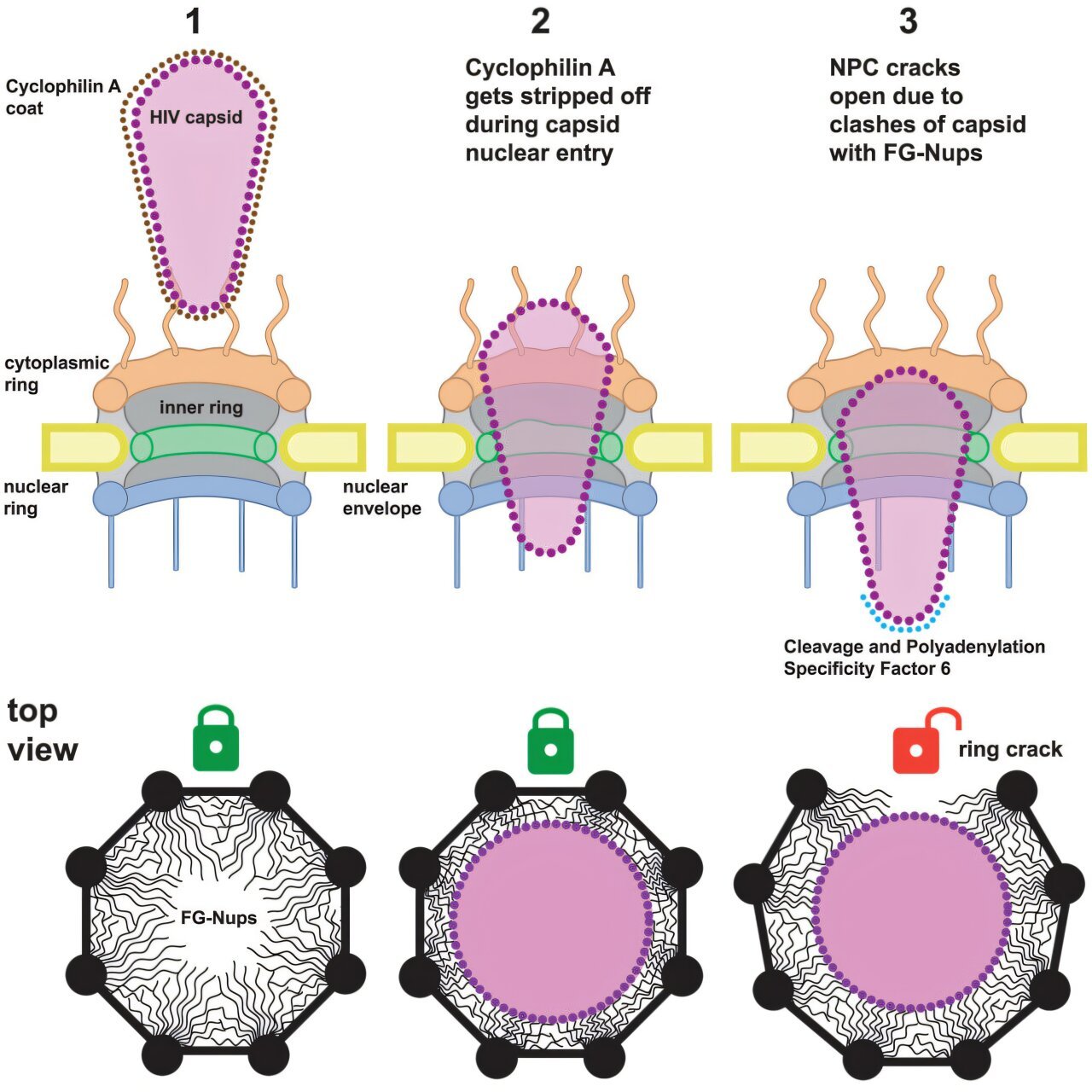
At the center of this intricate process is the HIV-1 capsid, a protein structure that protects the viral genome and aids in delivering it into the infected cell’s nucleus. The capsid is shaped uniquely—conically—formed by approximately 200 hexamers and pentamers of protein arranged in a mesh-like structure that resembles a football, though with a pointed, narrow end and a wider base. This structure is designed to encase the viral genetic material, ensuring its protection while it makes its journey through the host cell.
The successful infection process hinges on the capsid’s ability to open up and release its genetic contents once it reaches the host cell’s nucleus. To achieve this, it must first navigate through a critical barrier known as the nuclear pore complex (NPC), which regulates access to the cell’s nuclear compartment.
The Role of Nuclear Pores in Cellular Defense
The nuclear pore complex plays a pivotal role as the ‘guardians’ of the genome. These complexes form selective channels that span the nuclear envelope, regulating the passage of molecules between the cytoplasm and the nucleus. The NPCs are filled with specialized proteins known as FG-nucleoporins, which act as molecular bouncers. These proteins prevent the entry of most large or unnecessary molecules while allowing smaller ones or those with proper signals to pass through.
For a virus like HIV-1 to effectively infect a cell, it needs to find a way to bypass these guardians. Unlike most molecules that are excluded from the nuclear pore, the HIV-1 capsid can infiltrate this barrier, but how it does so was not fully understood until now.
The Path of HIV-1 Capsid Entry into the Nucleus
Previously, it was hypothesized that HIV-1 capsids would dissolve or release their viral content before they reached the nuclear pore, given the size constraints of the pore itself. At its widest dimension, the capsid is comparable in size to the diameter of the nuclear pore. It was assumed that this physical limitation meant the capsid would disintegrate or spill its contents before entering.
However, the team’s cutting-edge research using cellular tomography and super-resolution microscopy revealed a different story. Their high-resolution imaging uncovered that the capsids actually pass through the NPC with their narrow ends leading the way. As the capsid continues its journey into the nuclear pore, it does not collapse or decompose as expected. Instead, it moves deeper and deeper into the channel.
One of the key observations made was that the capsid does not deform as it travels. Rather, once the broad end of the cone-shaped capsid moves past the inner edge of the nuclear pore, it generates enough force to exert pressure on the surrounding proteins. As a result, many of the nuclear pores were observed to crack open at the location where the broad end of the capsid had penetrated.
The Force Behind Nuclear Pore Cracking
The discovery that the capsid can pass through the NPC without disintegrating represents a critical understanding of HIV’s entry process. The researchers suggest that, as the capsid moves through the pore with its broad end first, it generates a stretching force that causes the pore’s ring-shaped structure to crack, allowing the viral capsid to pass into the nucleus. It is compared to the effect of a nail driving into wood—once the force is applied, the surrounding material cracks open, widening the pore and allowing the passage of the capsid.
Computational simulations conducted by the research team support this hypothesis, revealing that the capsid could not pass through the pore unless the ring structure expanded or cracked. This insight leads to a fascinating suggestion: the evolution of the HIV-1 capsid’s unique conical shape may have developed specifically to break the nuclear pore complex, providing a mechanism for the successful delivery of the viral genome into the host’s nucleus.
The Impact of Capsid-Targeting Drugs
The breakthrough in understanding HIV’s interaction with the nuclear pore complex has practical implications for HIV treatment strategies. Over the last few decades, treatment options for HIV infection have improved dramatically. Lenacapavir, a drug recently granted FDA Breakthrough Therapy Designation for HIV Pre-exposure Prophylaxis (PrEP), targets the capsid to inhibit the release of the viral genome into the host cell, preventing HIV infection in individuals who use the drug.
Although effective in blocking HIV infection, lenacapavir does not reverse the integration of the viral genome into the host DNA. Therefore, while it can stop the spread of infection, it does not offer a cure for those who are already infected. Nonetheless, the continued development of drugs such as lenacapavir is a testament to the importance of understanding the virus’s mechanisms at a molecular level, as evidenced by the new research on capsid and nuclear pore interaction.
Lenacapavir and HIV Capsid Stabilization
An interesting facet of the study is how lenacapavir interacts with the HIV-1 capsid. Lenacapavir has been shown to stabilize the capsid, likely preventing its disassembly and the release of the viral genome. This stabilization further hinders the virus’s ability to replicate and infect new cells. In fact, lenacapavir’s function as a capsid-targeting agent brings the significance of this new molecular insight into sharper focus. The drug seems to prevent the capsid from opening entirely, stopping the virus at a critical juncture before it can inject its genetic payload into the host’s DNA.
Despite the advances in understanding HIV-1 capsid mechanics, there are still open questions. For example, it remains unclear whether the cracking of the nuclear pore provides a significant advantage to the virus, such as facilitating the delivery of a larger viral genome. Furthermore, the precise mechanisms by which the capsid finally opens within the nucleus to release its genetic content remain to be fully elucidated. This area of research will undoubtedly continue to be an important focus as scientists strive to uncover the final steps of the HIV-1 life cycle and how they might be interrupted to prevent infection.
Conclusion
This study marks an important milestone in HIV research, offering a more detailed understanding of a critical phase of the infection process—the entry of the HIV-1 capsid into the host cell nucleus. Through cutting-edge imaging techniques and sophisticated computational models, researchers have uncovered that HIV-1 exploits a unique mechanism to breach the nuclear pore, using its conical capsid to generate a force that cracks the pore open.
Such insights into the molecular intricacies of HIV-1’s entry open the door for more targeted interventions, such as drug development aimed at interrupting this process. Although treatments like lenacapavir show promise in preventing HIV infection, challenges remain, particularly regarding the virus’s ability to integrate its genome into the host’s DNA and remain undetected. Understanding the complete cycle of HIV’s life cycle—from its interaction with the host cell to its eventual release—will be crucial in the ongoing pursuit of a cure.
In the long run, this fundamental research contributes to the broader fight against HIV, offering hope that new therapies will emerge to combat this devastating virus, with the ultimate goal of eradication.
Reference: Jan Philipp Kreysing et al, Passage of the HIV capsid cracks the nuclear pore, Cell (2025). DOI: 10.1016/j.cell.2024.12.008