Engineers from The University of Warwick’s Integrative Synthetic Biology Center and Imperial College London’s Department of Bioengineering have recently achieved a significant milestone in the sustainable production of chemicals. These researchers have discovered ways to enhance microbial “cell-factories,” or engineered cells, to produce high-value chemicals more efficiently, challenging the traditional reliance on petrochemical-based processes. The breakthroughs not only promise to drive innovation in numerous industries—from pharmaceuticals to plastics—but also open a greener path for chemical production that could drastically reduce environmental impact.
The Need for a Greener Chemical Industry
Historically, the manufacturing of chemicals—especially those used in everyday products like household goods, clothing, and food—has relied heavily on fossil fuel-derived petrochemicals. These processes are carbon-intensive, contributing to significant environmental damage and greenhouse gas emissions. Given that about 14% of global greenhouse gases come from fossil-fuel-based chemical synthesis, finding alternative, more sustainable methods has become a pressing challenge for scientists and engineers worldwide.
One potential solution is biological production, which involves re-engineering microorganisms such as bacteria and yeast to act as “factories” that produce valuable chemicals from sustainable, low-cost feedstocks. The concept of using living cells as chemical factories holds promise for creating a more sustainable future by replacing traditional, energy-heavy processes. However, biological systems have their own challenges due to natural constraints within living organisms. To date, even though microbial production is cleaner, it has typically been less efficient than petrochemical processes. This is where the new advancements from The University of Warwick and Imperial College London come in.
Key Discoveries: Improving Microbial Efficiency
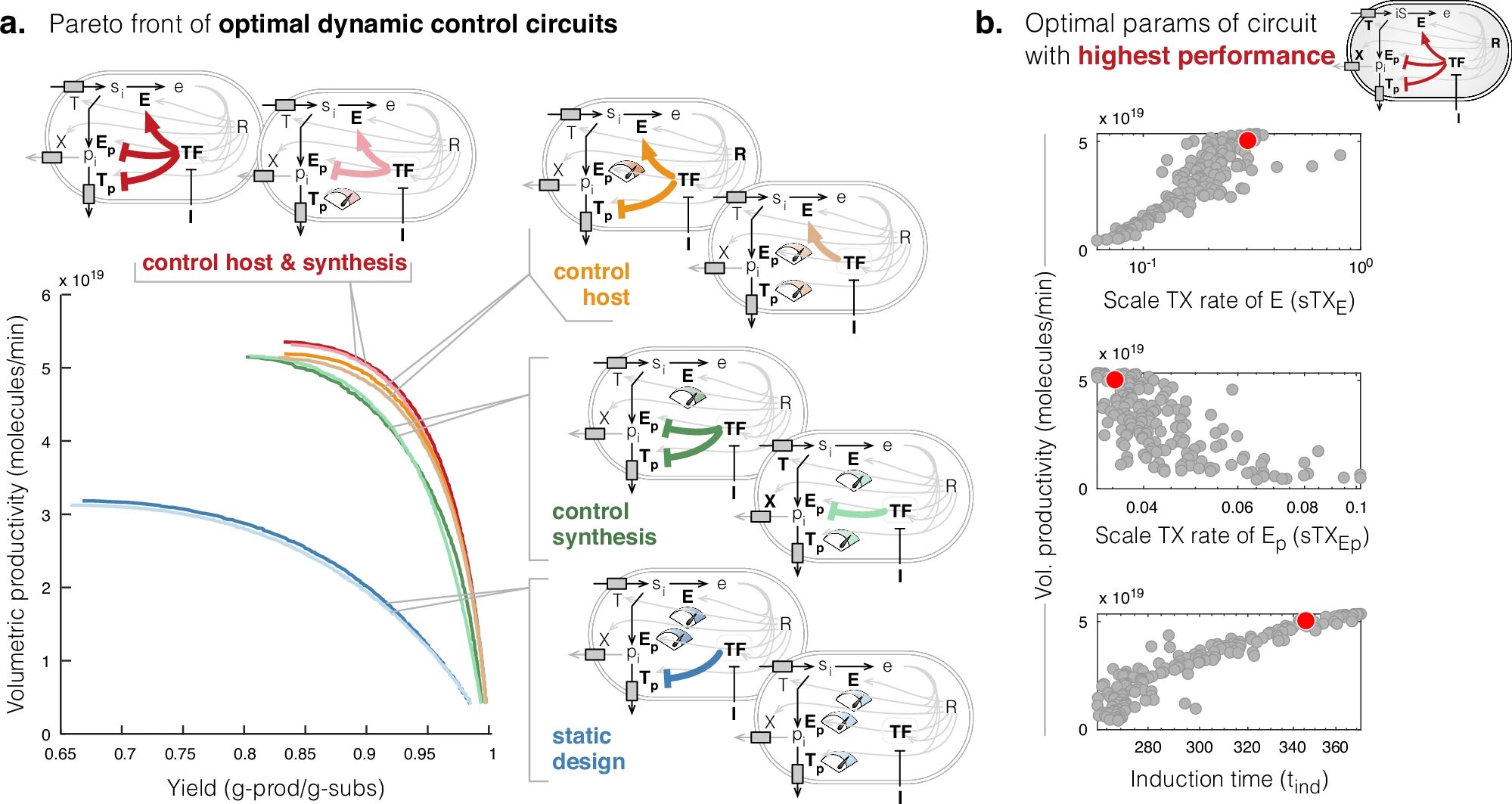
Using computational modeling, the team at Warwick and Imperial College has found a way to make minor but significant tweaks to current production systems, boosting the efficiency of bio-based chemical synthesis nearly two-fold. Their findings suggest that modest adjustments could make engineered microbes far more productive, opening the door to scalable bio-manufacturing processes.
Dr. Alexander Darlington, a Royal Academy of Engineering Research Fellow and Assistant Professor at The University of Warwick, shared the significance of their research: “Our research offers strategies for designing bacteria that are easier to implement than those currently considered state-of-the-art.” He explained that by testing around 500 different control mechanisms, they identified two novel approaches that were previously unexplored in bio-manufacturing. These innovations pave the way for a more efficient and economically viable route for producing essential chemicals sustainably.
One of the key insights from the research is that it is not just the synthesis of a product that matters but how growth and production processes are managed within cells. The team discovered that adjusting microbial design to prioritize chemical production over cell growth could maximize yield. Interestingly, instead of halting growth processes entirely in favor of synthesis, reprogramming cells to deprioritize growth—allowing them to grow large populations before switching to production—resulted in higher chemical output in the shortest time frame.
Boosting Yields through Nutrient Uptake and Process Adjustments
Another critical discovery centered around increasing the efficiency of nutrient uptake within the engineered cells. By tweaking cellular mechanisms to take in more nutrients, microbial factories can achieve even higher yields. The researchers suggest that reprogramming bacteria and yeast to optimize nutrient processing could deliver substantial increases in the quantity of the targeted chemicals, with relatively low adjustments to existing bioprocessing technologies.
The economic component of this study is also noteworthy. The team employed sophisticated econometrics, which is the use of mathematical models to simulate economic variables. The goal was to determine how manufacturers can design systems to maximize either productivity—achieving the fastest production times—or yield—producing more chemical from the same raw material. This flexibility makes the engineered microorganisms suitable for different market conditions. For instance, in markets where feedstocks are expensive, focusing on maximizing yield would be most cost-efficient, while higher productivity might be a priority if the chemical product is in high demand.
The Future of Bio-Based Chemical Production
The promise of engineered microbial factories extends far beyond just laboratory experiments. These innovations could significantly change the way chemicals are produced on an industrial scale. Microbial “cell factories” offer a renewable and sustainable alternative to fossil-fuel-driven methods of chemical synthesis. As the global chemical industry transitions toward greener practices, scaling up bio-based production could be a game-changer for sectors ranging from pharmaceuticals and agriculture to plastics and food.
As Dr. Ahmad Mannan, a postdoctoral researcher at Imperial College London, explained, “As an engineer, I want to minimize the negative impact our living has on others and the environment and achieve sustainable and renewable chemical production—and bacteria can facilitate that.” With expertise at the intersection of mathematics, molecular biology, and synthetic biology, Mannan and the team are uncovering simple yet profound biological principles that could drive economically viable chemical production for a wide variety of industrial applications.
Scaling Up: Industry Impact and Net Zero Goals
The ultimate goal for these research findings is to help industries adopt these new methods into their Research and Development (R&D) programs. Industry partners are currently working with the Warwick and Imperial teams to validate the strategies in practical, industrial environments. Once scaled, these bio-based cell-factories could not only meet current demands for chemicals but also pave the way for future production systems that are cleaner and more efficient.
The U.K. government’s target of reaching “net zero” by 2050 has placed additional pressure on industries to adopt sustainable technologies. Traditional petrochemical processes are a major contributor to greenhouse gas emissions, and if such bio-engineered systems were to replace fossil-fuel-based processes on a large scale, they could have a profound impact on the chemical sector’s carbon footprint. The incorporation of microbial cell factories as an alternative to conventional chemical synthesis methods could be one of the most important steps toward achieving a net-zero emissions target and fighting climate change.
Conclusion
This breakthrough represents a promising shift toward more sustainable chemical production methods. By applying principles of computational modeling, synthetic biology, and advanced bioengineering techniques, the team from The University of Warwick and Imperial College London has unlocked new pathways to significantly boost the efficiency of bio-based chemical manufacturing. With potential for higher productivity, greater yields, and economic feasibility, this research stands to revolutionize not just the chemical industry, but also play a key role in environmental conservation. Ultimately, these innovations could drastically reduce the dependency on fossil fuels, offering a cleaner, more sustainable path for creating the everyday chemicals we rely on.
Reference: Ahmad A. Mannan et al, Design principles for engineering bacteria to maximise chemical production from batch cultures, Nature Communications (2025). DOI: 10.1038/s41467-024-55347-y