Plastics are undeniably useful materials that have become an integral part of nearly every aspect of modern life. From packaging to electronics, transportation to healthcare, plastics are valued for their durability, versatility, and low cost. Yet, these very qualities that make plastics so useful also contribute to one of the biggest environmental challenges of our time. With global plastic production exceeding 400 million tons annually, the increasing consumption and disposal of plastic has led to widespread pollution. More concerning is the fact that only one-tenth of all plastic waste is recycled, leaving the vast majority to accumulate in landfills or end up in oceans, rivers, and other ecosystems.
As plastic waste continues to grow at an alarming rate, there is an urgent need for more effective and sustainable solutions for managing this waste. Traditional recycling methods have struggled to keep up with the sheer volume of plastic waste generated each year. While mechanical recycling techniques can reprocess plastics by melting and remolding them, this process often results in materials of lower quality, which limits their potential for reuse. Additionally, many types of plastic are difficult to recycle through traditional means, leaving large amounts of plastic waste that cannot be reused or recycled.
This has led to the emergence of catalytic recycling, an innovative approach to tackling plastic waste that could provide a much-needed solution. Unlike conventional recycling, which focuses on physical transformation, catalytic recycling involves breaking down plastics into simpler components using chemical catalysts. The process can convert plastic waste into valuable chemicals and fuels, such as gasoline and diesel, offering the potential for a more sustainable, efficient, and profitable recycling method. While catalytic recycling shows great promise, it is still a relatively young technology that requires further refinement before it can be scaled up for industrial use.
Catalytic Recycling: The New Frontier in Plastic Waste Management
Catalytic recycling processes, such as hydrogenolysis and hydrocracking, use catalysts to break the long polymer chains of plastics into smaller, more useful components. These processes can be particularly beneficial for dealing with polyolefins, a class of plastics that includes commonly used materials like polyethylene (PE) and polypropylene (PP). Polyolefins make up around 55% of global plastic waste, making them a prime target for catalytic recycling innovations.
Traditional catalytic recycling methods have struggled to achieve the high efficiency required for large-scale use, with many processes suffering from low conversion rates and the formation of unwanted by-products like coke, a carbon-rich residue that can deactivate catalysts and reduce the process’s efficiency. The challenge, then, has been to develop catalysts that can effectively break down polyolefins while minimizing the production of coke and maximizing the yield of valuable chemicals and fuels.
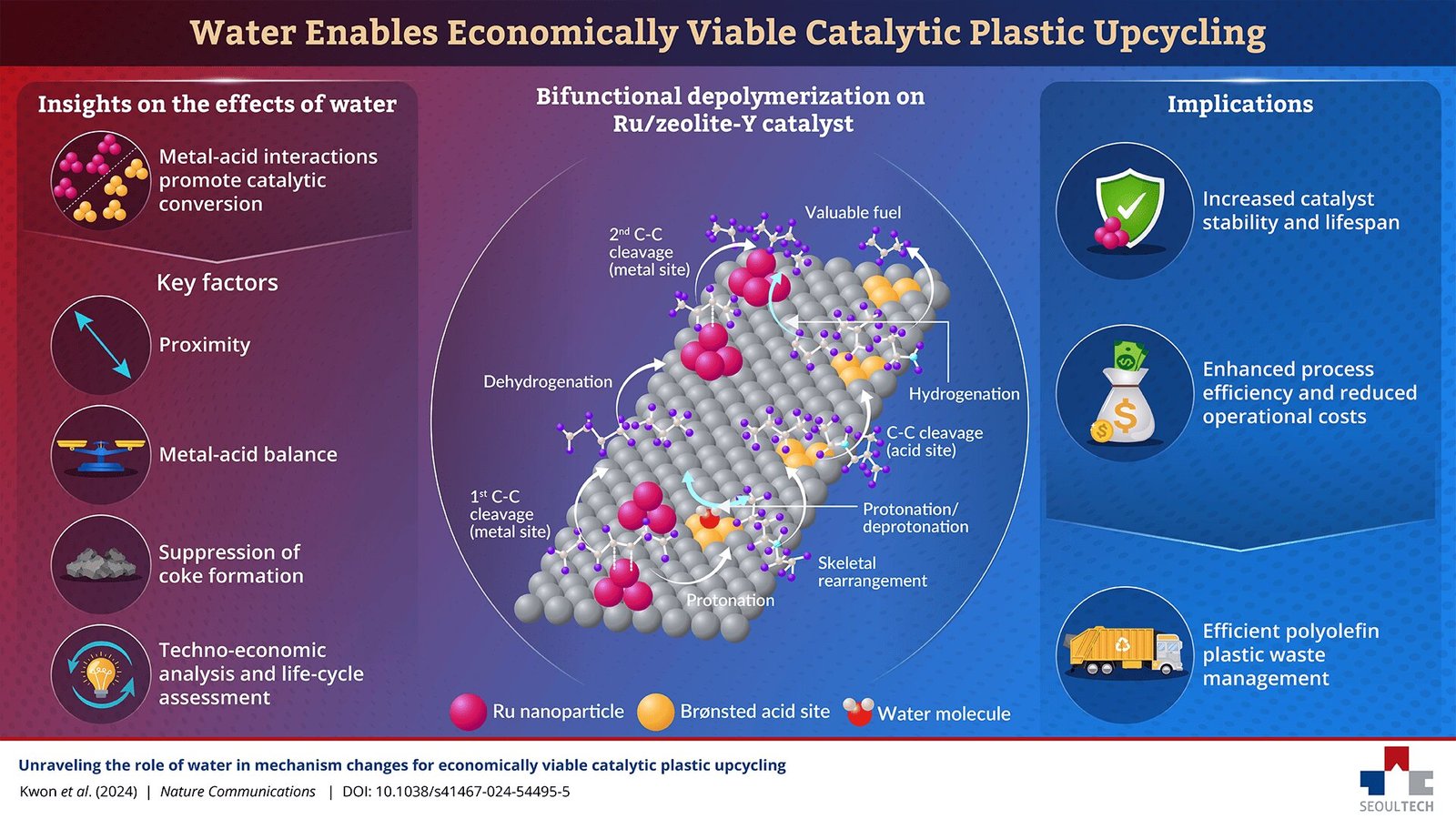
This is where recent research led by Professor Insoo Ro from the Seoul National University of Science and Technology (South Korea) comes into play. In a groundbreaking study published in Nature Communications on November 29, 2024, Ro and his research team unveiled a novel approach to catalytic recycling that significantly improves the efficiency of polyolefin depolymerization. By adding water to the reaction mixture when using ruthenium (Ru)-based catalysts, the team was able to enhance the conversion rates of polyolefins while reducing coke formation—a major issue in catalytic recycling processes.
The Breakthrough: Water-Enhanced Catalytic Depolymerization
One of the key findings of this study is the surprising role that water can play in catalytic recycling. While it is well known that water is often used as a solvent in chemical reactions, its presence in this case appears to alter the reaction mechanisms in a way that promotes more efficient depolymerization of polyolefins. By adding water, the Ru-based catalysts exhibited a dramatic improvement in conversion rates and catalytic activity, leading to higher yields of valuable products such as fuels and chemicals.
In particular, the research team focused on catalysts that had both metal sites (for hydrogenation) and acidic sites (for promoting bond cleavage). The presence of water in the reaction mixture seemed to activate these metal and acid sites more effectively, facilitating the breakdown of polyolefins into smaller molecules while suppressing coke formation. This is a significant advancement because coke buildup is one of the main challenges faced by catalytic processes. By reducing coke formation, the process becomes more efficient, the catalyst’s lifespan is extended, and the operational costs are lowered.
As Dr. Ro explained, “The addition of water alters the reaction mechanisms, promoting pathways that enhance catalytic activity while suppressing coke formation. This dual role improves process efficiency, extends catalyst lifespan, and reduces operational costs.” This breakthrough represents a significant step forward in catalytic recycling, offering a solution to some of the key obstacles that have hindered the adoption of this technology in industrial settings.
Optimizing Catalysts for Maximum Efficiency
To further optimize the catalytic recycling process, the research team conducted an in-depth study of the reaction mechanisms and the role of catalyst composition. They experimented with different Ru-based catalysts supported on various materials, including zeolite—a porous, crystalline material commonly used in catalysis. Their findings revealed that Ru/zeolite-Y catalysts performed exceptionally well in polyolefin depolymerization, achieving a remarkable 96.9% conversion rate under optimal conditions. This high conversion rate is crucial for making catalytic recycling a viable alternative to traditional waste management practices.
The team also examined the effect of ruthenium (Ru) content and the proximity and balance between metal and acid sites on the catalyst’s performance. They found that an optimal balance between these factors significantly enhanced catalytic efficiency, suggesting that careful tuning of catalyst composition is essential for maximizing the performance of catalytic recycling processes.
Techno-Economic and Environmental Benefits
To assess the feasibility of scaling up this new catalytic recycling approach, the research team conducted a techno-economic analysis and a life cycle assessment of the proposed process. These analyses are critical for understanding whether the technology can be commercially viable and environmentally sustainable on a large scale.
The results of the analysis were promising. The addition of water not only increased the carbon efficiency of the process but also improved its economic and environmental performance. By converting polyolefins into valuable fuels like gasoline and diesel, the process could help reduce the reliance on fossil fuels while also addressing the growing plastic waste crisis. Additionally, by offering an alternative to conventional waste management practices, this method could significantly reduce the amount of plastic waste that ends up in landfills or oceans, helping to combat the global plastic pollution problem.
Dr. Ro highlighted the importance of this discovery, stating, “The addition of water not only enhances carbon efficiency, it improves economic and environmental performance, also increases the conversion of polyolefins to valuable fuels like gasoline and diesel. This approach thus represents a viable alternative to conventional waste management practices and offers a solution to reduce landfill and ocean pollution caused by polyolefins—the largest contributor to plastic waste.”
A Sustainable Future for Plastic Waste Recycling
The potential of this catalytic recycling method extends beyond just improving the efficiency of polyolefin conversion. In the long term, it could provide a solution to one of the most pressing environmental challenges of our time—plastic pollution. As plastic waste continues to accumulate, finding ways to recycle it effectively and sustainably is crucial to preserving the health of our planet.
The research team is optimistic about the future of this technology. They envision a world where catalytic recycling processes like the one they’ve developed can be scaled up to handle mixed plastic waste, eliminating the need for pre-sorting and making recycling efforts more cost-effective and easier to implement. Such advancements could help drive policy changes, inspire investment in advanced recycling infrastructure, and foster international collaborations to tackle the global plastic waste crisis.
“By demonstrating a sustainable and economic approach to transforming plastic waste into valuable resources, our research could help drive policy changes, inspire investment in advanced recycling infrastructure, and foster international collaborations to address the global plastic waste crisis. Over time, these advancements promise cleaner environments, reduced pollution, and a more sustainable future,” concludes Dr. Ro.
Conclusion: A Step Toward a Cleaner, More Sustainable Future
This breakthrough in catalytic depolymerization marks a significant advancement in the quest to solve the global plastic waste crisis. By developing a more efficient, sustainable, and economically viable method for recycling polyolefins, Dr. Ro and his team have opened the door to a future where plastic waste can be transformed into valuable resources instead of polluting the environment. As the technology continues to evolve, it holds the potential to revolutionize the way we manage plastic waste and contribute to a cleaner, more sustainable world.
Reference: Taeeun Kwon et al, Unraveling the role of water in mechanism changes for economically viable catalytic plastic upcycling, Nature Communications (2024). DOI: 10.1038/s41467-024-54495-5