Late-onset Alzheimer’s disease is a debilitating neurodegenerative disorder that affects millions of people worldwide, with the number of affected individuals set to nearly double by 2050. In the United States alone, the Alzheimer’s Association estimates that around 7 million Americans are currently living with Alzheimer’s, and this number is projected to rise to 13 million by mid-century. Despite its widespread impact, the exact causes of Alzheimer’s remain complex and multifactorial, and while there is no cure for the disease, significant strides in early detection and intervention can help slow its progression and preserve cognitive function for longer.
One of the most promising recent breakthroughs in Alzheimer’s research comes from a study conducted by researchers at The University of Texas Health Science Center at San Antonio (UT Health San Antonio). Published in Molecular Neurodegeneration, the study identifies a specific protein-coding transcript that may contribute to Alzheimer’s risk across all genetic types of the apolipoprotein E (APOE) gene. This discovery could open new doors for understanding how genetic factors influence Alzheimer’s risk and lead to the development of more effective diagnostic tools and therapeutic interventions.
Understanding APOE and its Variants
The APOE gene plays a central role in the development of Alzheimer’s disease. All humans inherit two APOE alleles, one from each parent. These alleles are classified into three major types: ε2, ε3, and ε4. APOE ε3 is the most common and is considered neutral in terms of Alzheimer’s risk. In contrast, APOE ε2 has been found to offer some protective effects against Alzheimer’s, while APOE ε4 is associated with a higher risk of developing the disease.
However, the mere presence of the APOE ε4 allele does not guarantee the onset of Alzheimer’s disease. Many people carrying this allele may never develop the condition, while others without APOE ε4 may still succumb to Alzheimer’s. This complexity has prompted researchers to explore additional genetic factors that may contribute to Alzheimer’s risk beyond the APOE gene alone.
Agustin Ruiz, MD, Ph.D., a co-investigator of the study and professor at the Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases at UT Health San Antonio, highlights the difficulty in fully understanding how APOE ε4 elevates Alzheimer’s risk. He emphasizes that APOE gene expression is regulated in various tissues, organs, and cell types throughout the body, and understanding this regulation is crucial for uncovering the gene’s role in diseases like dementia. “Despite over three decades of dedicated research, the precise mechanism by which the APOE ε4 allele elevates the risk of Alzheimer’s disease and related dementias remains elusive,” Ruiz explains.
Insights from Diverse Brain Studies
Recent genetic studies have uncovered several variants in the APOE region that may increase Alzheimer’s risk, yet the challenge remains in pinpointing exactly which APOE elements these variants influence, particularly in the brain. In this study, Ma’s research team conducted an in-depth analysis of five datasets that included data from more than 1,000 postmortem human brains of individuals of both European and African ancestry. The researchers focused on three brain regions, with particular emphasis on the prefrontal cortex—a crucial area of the brain responsible for learning, memory, and executive functions.
The inclusion of brains from diverse populations is vital because the prevalence, progression, and risk factors for Alzheimer’s disease can vary across different ethnic groups. By studying brains from both European and African ancestries, the researchers were able to identify common genetic risk factors that may apply to a broader range of populations. Ma, one of the lead researchers, underscores the importance of diversifying genetic studies to gain a comprehensive understanding of Alzheimer’s risk. “We need brain data from other populations so we can compare it to data we have for Europeans,” he says, adding that future research should aim to include Asian, Hispanic, and other populations to get a fuller picture of the genetic factors involved in Alzheimer’s.
What Are Transcripts and Single Nucleotide Polymorphisms?
Understanding the complex relationship between genes and disease involves studying messenger RNA (mRNA), which acts as a blueprint for protein production. Genes like APOE produce mRNA, which is then translated into proteins, essential molecules that carry out various functions in the body. The regulation of mRNA expression can have a significant impact on disease risk, and alterations in how mRNA is processed may contribute to the development of diseases such as Alzheimer’s.
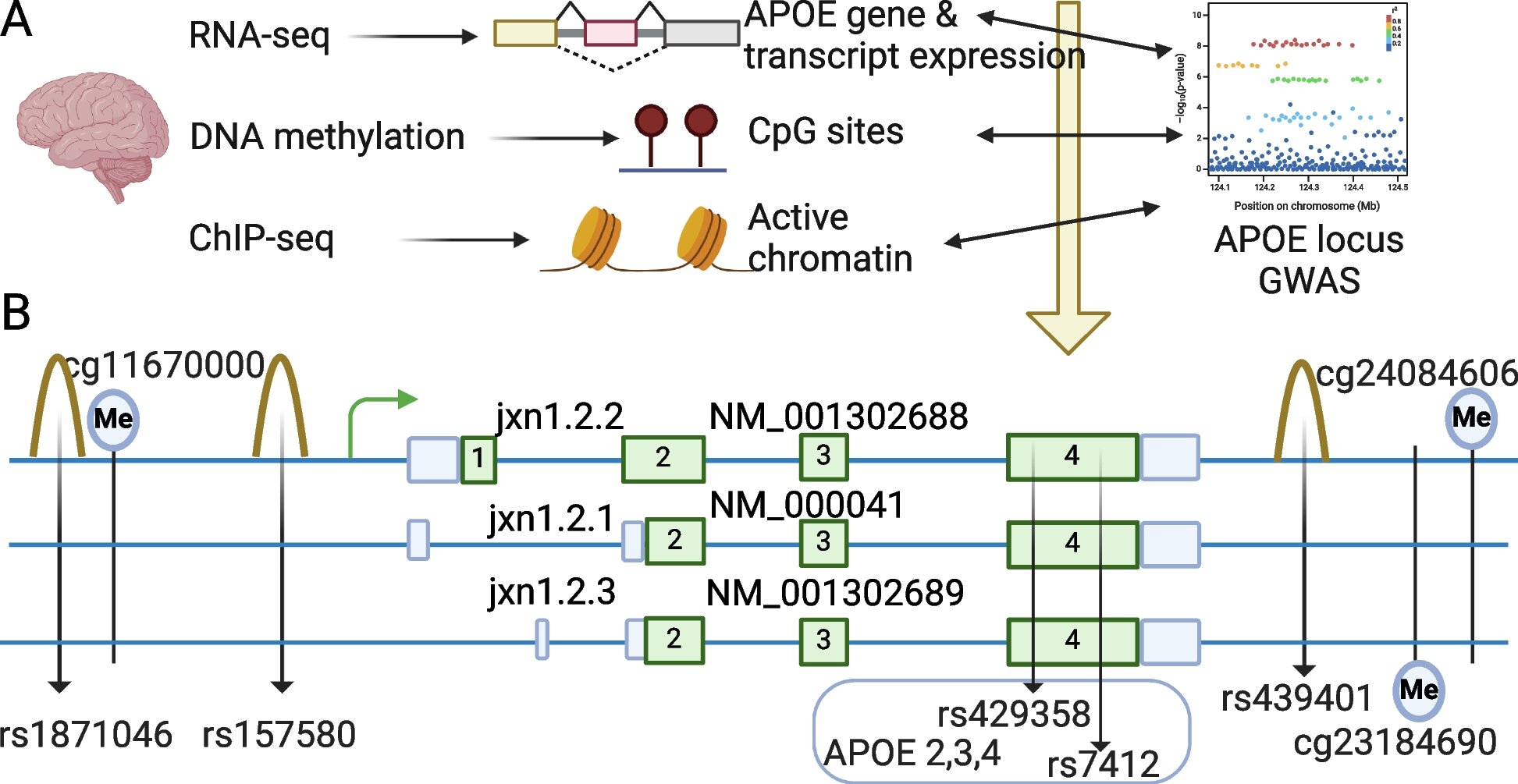
In the study conducted by UT Health San Antonio, the researchers focused on a specific mRNA transcript linked to the APOE gene, known as jxn1.2.2. This transcript appeared to be dysregulated in the prefrontal cortex of individuals with Alzheimer’s disease, suggesting that it may contribute to the risk of developing the condition. Importantly, this dysregulation was observed in both European and African brain samples, regardless of whether the individual carried the APOE ε2, ε3, or ε4 allele.
The researchers also examined single nucleotide polymorphisms (SNPs), which are common genetic variations that occur when a single nucleotide in the DNA sequence is altered. While most SNPs are harmless, some can influence gene expression and contribute to disease risk. Two particular SNPs—rs157580 and rs439401—were found to be associated with the dysregulation of APOE transcript jxn1.2.2, and these variations appeared to have consistent effects across both European and African populations.
Interestingly, these SNPs were also associated with amyloid-beta and phosphorylated tau, two key biomarkers for Alzheimer’s disease. Amyloid-beta proteins can form clumps, creating plaques in the brain, while tau proteins become tangled, disrupting the function of neurons. Both of these processes are central to the pathology of Alzheimer’s disease, and their connection to these genetic variants strengthens the case for jxn1.2.2 as a potential contributor to disease risk.
The Potential for Cross-Population Therapeutics
One of the most promising aspects of this research is that the risk factors identified—particularly the changes in APOE transcript jxn1.2.2—appear to be consistent across different populations. This is important because it suggests that therapies targeting this genetic element could have broad applicability, potentially benefiting both European and African American populations, as well as other groups.
As Ma explains, “The data shows that the risk factor, gene expression changes, and polymorphisms are consistent between European and African ancestries. This suggests these are common risk factors across both populations. That’s good news, because if we develop a drug, we can potentially use it to treat both populations.” This finding increases the potential for developing universal treatments that could address Alzheimer’s across diverse populations.
Implications for Diagnosis and Treatment
The discovery of APOE transcript jxn1.2.2 as a potential genetic risk factor for Alzheimer’s disease marks a significant step forward in the understanding of the disease’s complex genetic landscape. By uncovering the role of this transcript in the brain, researchers are one step closer to identifying reliable biomarkers for Alzheimer’s. These biomarkers could lead to earlier detection and provide a means for monitoring disease progression, ultimately enabling more effective interventions.
Moreover, the research highlights the importance of considering genetic diversity in Alzheimer’s research. By analyzing diverse populations, scientists can uncover risk factors and therapeutic targets that might otherwise go unnoticed in studies focused solely on one ethnic group. The ultimate goal is to develop personalized treatments that address the underlying mechanisms of Alzheimer’s disease, offering hope for better management and prevention of this devastating condition.
Dr. Ruiz emphasizes that this discovery opens new avenues for exploring the molecular mechanisms behind Alzheimer’s. “This discovery not only enhances our comprehension of APOE function in the brain but also paves the way for novel research avenues into the underlying mechanisms of Alzheimer’s disease. Deciphering the physiological role of this transcript and its potential contribution to disease progression is a current challenge that holds promise for the development of innovative therapeutic strategies.”
Conclusion
As the global population ages, Alzheimer’s disease continues to pose an immense challenge to public health. However, new research like the study from UT Health San Antonio provides hope that the complexities of the disease may eventually be unraveled. The identification of genetic factors such as APOE transcript jxn1.2.2 brings us closer to developing better diagnostic tools, treatments, and preventive measures. By focusing on genetic diversity and exploring the multifaceted nature of Alzheimer’s risk, researchers are forging a path toward a future where Alzheimer’s disease can be managed more effectively, if not entirely prevented.
Reference: Qiang Chen et al, Identification of a specific APOE transcript and functional elements associated with Alzheimer’s disease, Molecular Neurodegeneration (2024). DOI: 10.1186/s13024-024-00751-7