Alzheimer’s disease, a progressive neurodegenerative disorder that causes memory loss, confusion, and changes in behavior, has long been a focus of scientific research. One of the most prominent theories about its development has been the amyloid cascade hypothesis, which suggests that a buildup of amyloid beta (Aβ) proteins in the brain triggers a series of events leading to neurodegeneration. While the buildup of Aβ remains central to Alzheimer’s research, a new study has provided fresh insights into a possible underlying cause for the disease: stalled protein processing in the brain. This research could pave the way for new drug development and therapeutic strategies aimed at treating Alzheimer’s disease.
The study, published today in eLife, is described by its editors as a fundamental breakthrough in understanding how mutations in the presenilin-1 (PSEN1) gene affect the processing of amyloid precursor protein (APP). APP is a large protein that, when processed incorrectly, produces amyloid beta (Aβ) protein, which has been found to accumulate in the brains of Alzheimer’s patients. The research could provide compelling new avenues for Alzheimer’s drug development, focusing on the process of protein production rather than just the end-product, amyloid beta.
Unpacking the Amyloid Cascade Hypothesis
For decades, scientists have focused on the amyloid cascade hypothesis, which suggests that the accumulation of Aβ protein in the brain is the initiating event in Alzheimer’s disease. According to this hypothesis, the buildup of Aβ aggregates leads to a cascade of toxic effects, including inflammation, cell death, and damage to brain structures involved in memory and cognition.
Despite the widespread acceptance of this hypothesis, researchers have struggled to fully understand how the aggregation of Aβ proteins begins, and why this process is so damaging. Clinical trials targeting Aβ aggregates have not yielded the breakthrough treatments scientists hoped for, leading to a reconsideration of the hypothesis as the sole cause of Alzheimer’s. The limitations of amyloid-targeting therapies have spurred scientists to explore other potential causes of the disease, including the process of protein proteolysis, during which APP is cleaved by enzymes to produce smaller fragments.
Proteolysis and Its Role in Alzheimer’s Disease
Proteolysis is the process by which larger proteins are broken down into smaller functional fragments. In Alzheimer’s disease, this process is particularly important because the amyloid precursor protein (APP) is typically cleaved by the gamma-secretase (γ-secretase) enzyme. This enzyme trims the APP into several smaller fragments, one of which is the amyloid beta protein (Aβ). In a healthy brain, these Aβ proteins are produced in relatively small quantities and do not accumulate to dangerous levels. However, when this process goes awry, Aβ can accumulate, forming plaques that disrupt communication between neurons and contribute to neurodegeneration.
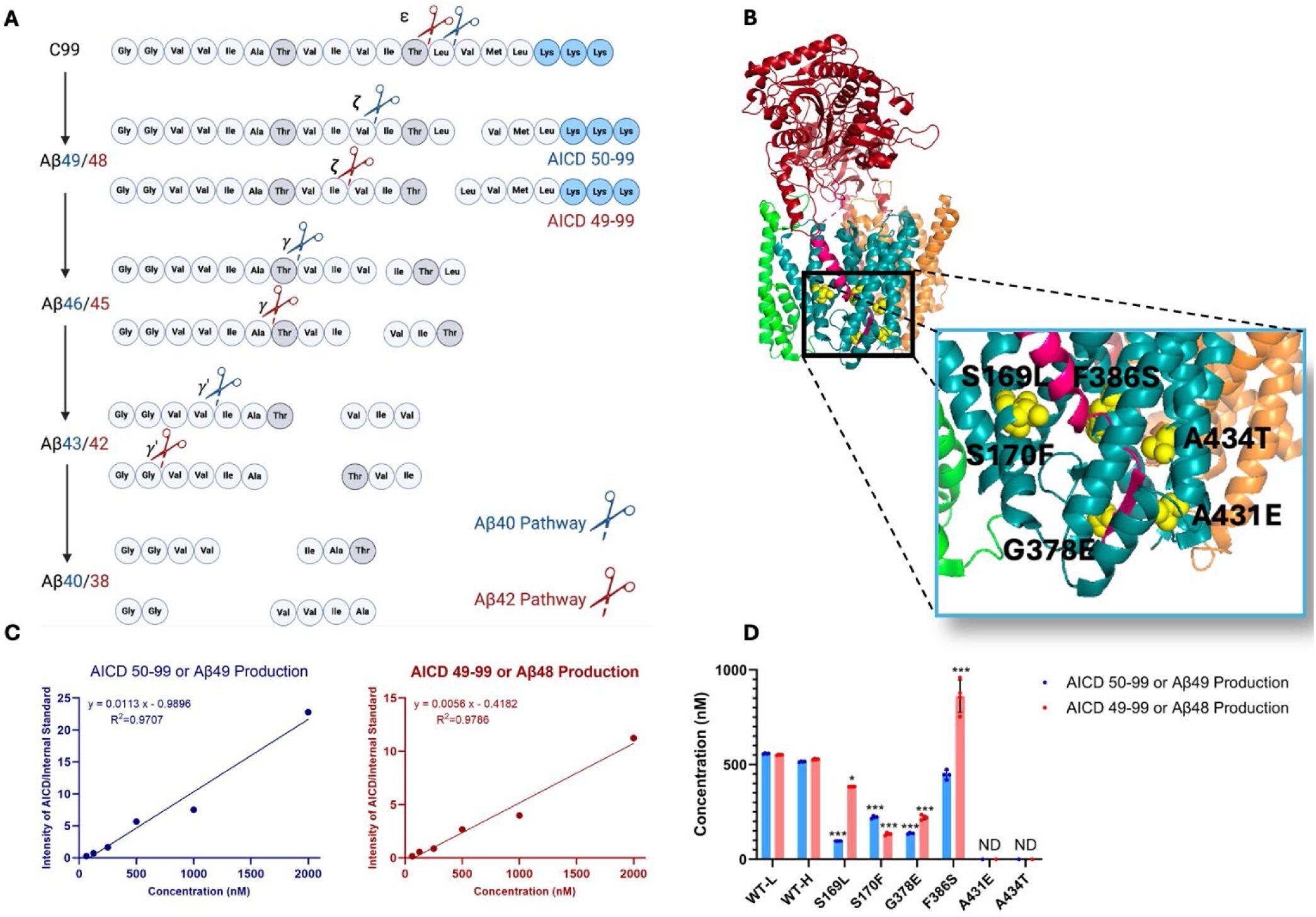
The current study, led by Parnian Arafi, a Medicinal Chemistry Research Assistant at the University of Kansas, takes a deeper look at how mutations in the PSEN1 gene affect the normal processing of APP. PSEN1 mutations are known to cause early-onset familial Alzheimer’s disease (FAD), a rare form of the disease that typically manifests between the ages of 27 and 58. These mutations interfere with the functioning of γ-secretase, impairing its ability to properly cleave APP, leading to a buildup of longer, more toxic intermediates in the processing pathway.

Stalled Proteolysis and Neurodegeneration
The study expands on previous research by Michael Wolfe, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, and colleagues, who demonstrated that mutations in PSEN1 lead to a stalling of γ-secretase-APP complexes in a worm model of Alzheimer’s disease. These stalled complexes, even in the absence of amyloid beta, were found to trigger neurodegeneration. This led the researchers to propose the hypothesis that stalled proteolysis—the failure to properly process APP—may be a key factor in Alzheimer’s disease, independent of the traditional amyloid cascade.
In the current study, the team further explored this hypothesis by investigating six additional mutations associated with early-onset familial Alzheimer’s disease. These mutations are of particular interest to the Dominantly Inherited Alzheimer Network (DIAN), a research initiative focused on studying the genetic causes of Alzheimer’s in families with a history of early-onset disease.
Experimental Approach and Findings
To investigate the effects of these mutations, the research team generated mutant γ-secretase enzymes and incubated them with an APP fragment to observe how the mutant enzymes processed the protein. They used mass spectrometry to analyze the resulting fragments and assess how each mutation altered the cleaving process.
What they found was troubling: all the mutations tested caused significant deficiencies in APP processing, with each mutation disrupting different stages of the proteolytic process. Some mutations led to a build-up of longer APP intermediates, while others caused the accumulation of abnormal Aβ fragments. Importantly, the researchers also found that these mutations stabilized the enzyme-substrate complexes—the point at which the γ-secretase enzyme binds to APP and begins processing it.
Using fluorescently labeled antibodies, the researchers were able to measure the proximity of the enzyme and its substrate. The results showed that, for all mutations, the fluorescent signal was reduced, indicating that the enzyme-substrate complexes were more stable and persisted longer than they should have. This suggests that the proteolysis process was stalled, preventing the proper breakdown of APP and allowing toxic intermediates to accumulate.
The Stalled Complex Hypothesis
The findings support the research team’s “stalled complex” hypothesis, which posits that the accumulation of these intermediate complexes—rather than Aβ alone—could be driving the neurodegeneration seen in Alzheimer’s disease. In this scenario, the buildup of incomplete APP fragments, held together by an overactive γ-secretase enzyme, disrupts normal cellular function and leads to neuronal death.
“We’ve shown that these mutations lead to stalled proteolysis and stabilize the enzyme with its substrate in an intermediate form,” says Arafi. “These findings are in keeping with our stalled complex hypothesis, where it is these enzyme-substrate complexes that trigger neurodegeneration even in the absence of amyloid beta-protein production.”
Implications for Alzheimer’s Disease Treatment
The implications of this research could be far-reaching. By identifying stalled proteolysis as a potential driver of Alzheimer’s, the study opens the door to new therapeutic approaches that go beyond targeting amyloid beta plaques.
“Difficulty in pinpointing the drivers of Alzheimer’s disease and in discovering effective therapeutics suggests that entities and processes beyond amyloid beta-protein might play pivotal roles in neurodegeneration,” explains Wolfe.
The researchers propose that targeting γ-secretase activators could offer a promising approach to treating Alzheimer’s. By rescuing stalled proteolysis and restoring normal APP processing, these activators might help prevent the accumulation of toxic intermediates and reduce the risk of neurodegeneration. These drugs could complement existing treatments aimed at other Alzheimer’s-related pathways, such as amyloid beta aggregation, and may offer a more comprehensive treatment strategy for the disease.
The focus on familial Alzheimer’s disease has also provided a more controlled environment for understanding the mechanisms that drive neurodegeneration, making it easier to identify pathogenic processes. This targeted approach is likely to lead to more specific and effective treatments for patients with early-onset Alzheimer’s, though the findings may eventually have broader applications for the more common late-onset form of the disease.
Conclusion
This study represents a significant step forward in understanding the complex mechanisms underlying Alzheimer’s disease. While amyloid beta has long been the focus of Alzheimer’s research, the discovery of stalled proteolysis as a potential cause of neurodegeneration highlights the importance of exploring new pathways. By targeting proteolysis dysfunction and developing therapies that rescue stalled processing, scientists could offer new hope for those affected by this devastating disease.
As researchers continue to investigate the role of protein processing in Alzheimer’s, this work provides a fresh perspective on how genetic mutations and disrupted cellular processes contribute to the disease. With continued collaboration and rigorous research, we may one day have more effective treatments—and, eventually, a cure—for Alzheimer’s disease.
Reference: Parnian Arafi et al, Alzheimer-mutant γ-secretase complexes stall amyloid β-peptide production, eLife (2025). DOI: 10.7554/eLife.102274.2