Researchers at the University of Waterloo have uncovered a promising solution for combating chromium contamination—an environmental issue that poses significant health risks. A special form of biochar, produced by heating agricultural waste without oxygen, has shown remarkable potential in absorbing toxic chromium(VI) and transforming it into its safer counterpart, chromium(III). This breakthrough could have far-reaching implications for cleaning up chromium pollution at industrial sites.
Chromium: A Dual-Natured Heavy Metal
Chromium is a heavy metal that exists in two primary forms: chromium(III) and chromium(VI). Chromium(III) is an essential micronutrient required in trace amounts by the human body for healthy metabolism. However, the more hazardous form, chromium(VI), is a well-known carcinogen. Exposure to chromium(VI) has been linked to lung, liver, and ovarian cancers, as well as reproductive problems. This toxic form is most commonly produced during industrial processes such as leather tanning, the production of stainless steel, and mining activities. Interestingly, chromium(VI) can also form naturally in areas with manganese-rich minerals, further complicating efforts to manage and mitigate its environmental impact.
Given its toxic effects, chromium(VI) contamination in water and soil is a significant concern for public health and environmental safety. Efforts to clean up chromium contamination have focused on developing effective and efficient methods for removing chromium from contaminated sites, particularly in water.
Biochar: A Natural Filter for Chromium
One promising solution is biochar, a form of charcoal produced by heating organic materials, such as agricultural waste, at high temperatures in the absence of oxygen. Biochar is rich in organic carbon, which gives it excellent properties as a filter, capable of absorbing various pollutants, including heavy metals like chromium. Researchers have been exploring biochar’s potential to remove toxic substances from contaminated water, harnessing its natural ability to adsorb pollutants.
Filip Budimir, a Ph.D. candidate in Earth and Environmental Sciences at the University of Waterloo, set out to explore how biochar could be used to mitigate chromium(VI) contamination in water. His groundbreaking research, published in the journal Chemosphere, focuses on the interaction between biochar and chromium(VI) in contaminated water, specifically using oak-based biochar as the filtering agent.
The Research: How Biochar Transforms Chromium
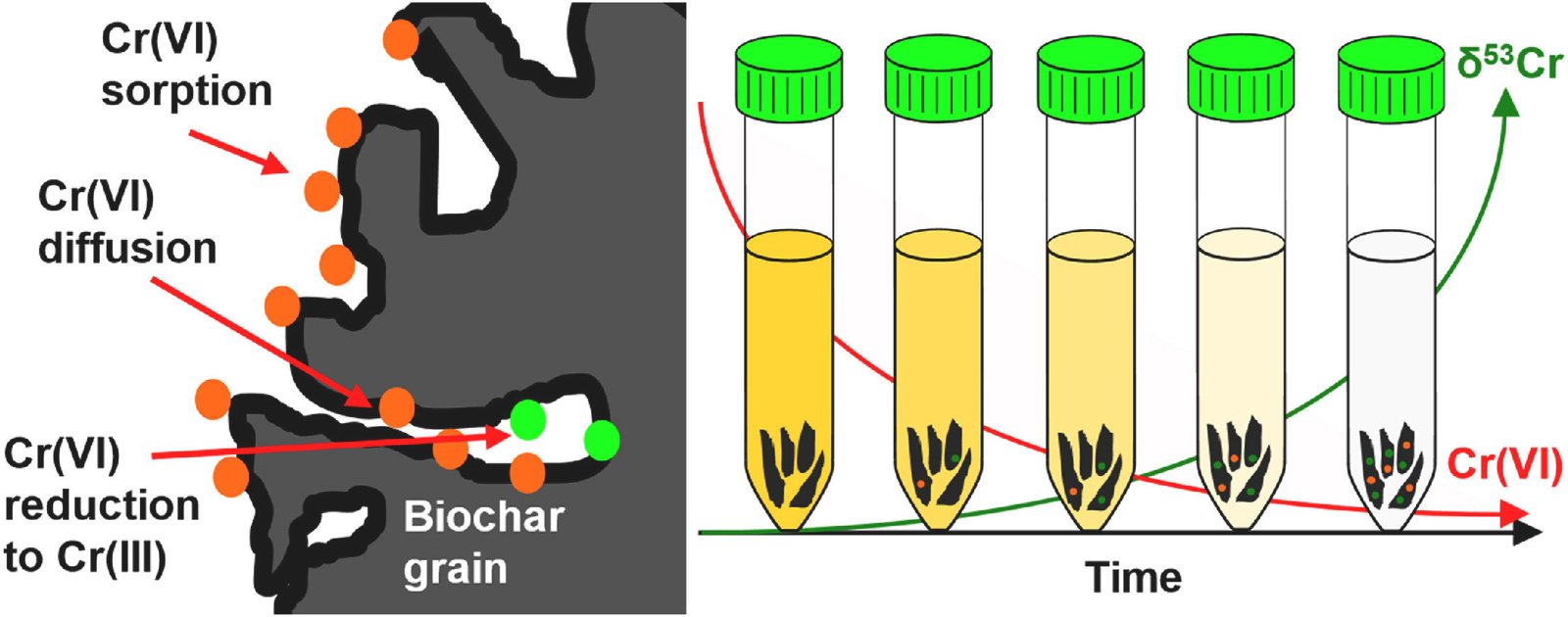
Budimir’s study aimed to determine what happens when water contaminated with chromium(VI) is mixed with oak-based biochar. To investigate this, he used advanced techniques at the Canadian Light Source at the University of Saskatchewan to probe the biochar at a molecular level. Using specialized equipment, Budimir could identify where the chromium was deposited on the biochar grains and determine which form of chromium was present.
The results were promising. In the initial phase, the water contained only chromium(VI), but after 120 hours (or five days), Budimir found that approximately 85% of the chromium had been converted to the safer chromium(III). This significant transformation suggests that not only was the biochar absorbing the toxic chromium from the water, but it was also actively converting it into a less harmful form.
“We were happy to see that the majority of what we were finding on the biochar grains was chromium-3 and not chromium-6,” said Budimir, highlighting the success of the biochar in detoxifying the contaminated water.

Isotope Fractionation and Monitoring Groundwater Remediation
Budimir’s study also revealed an interesting phenomenon: the isotopes of chromium underwent fractionation during the conversion process. Isotope fractionation refers to the change in the ratio of different isotopes of an element during a chemical process. In this case, the lighter chromium isotopes were removed from the water more quickly and converted from chromium(VI) to chromium(III) more readily than the heavier isotopes.
This discovery has important implications for monitoring efforts aimed at groundwater remediation. By analyzing the isotopic signatures of chromium in the water, researchers and environmental engineers could gain a deeper understanding of the effectiveness of the biochar-based filtration process.
“Things are happening underground, but we’re not sure what,” said Budimir. “Testing the isotopes can give us an idea of what is happening and if the process is working.”
The ability to track the isotopic changes in chromium during cleanup efforts could provide real-time insights into the progress of chromium removal and its conversion into a safer form. This could be a crucial tool for ensuring that groundwater contamination is being effectively addressed and for monitoring the long-term success of environmental remediation projects.
The Future of Biochar in Environmental Cleanup
Budimir’s findings open up new possibilities for using biochar as an effective tool in the fight against chromium contamination. The ability of biochar to absorb and transform toxic chromium(VI) into its safer chromium(III) form makes it an attractive option for cleaning up polluted sites, particularly in industries where chromium(VI) is a byproduct.
Given that biochar can be produced from abundant agricultural waste, it also presents a sustainable and cost-effective solution. This makes it a promising alternative to traditional chemical treatments, which can be expensive and often involve harmful substances.
The research also has broader implications for the use of biochar in the treatment of other environmental contaminants. Biochar has been shown to be effective in removing a wide range of pollutants, including heavy metals, organic compounds, and even greenhouse gases. As more studies are conducted, biochar’s potential as an environmental remediation tool will continue to expand.
Conclusion
The discovery by Filip Budimir and his team at the University of Waterloo that oak-based biochar can absorb and convert toxic chromium(VI) into its safer chromium(III) form is a significant step forward in the field of environmental science. This breakthrough offers a promising new approach to dealing with chromium contamination, particularly in water, and could be an effective tool for cleaning up industrial pollution. Moreover, the ability to monitor the isotopic changes in chromium during the filtration process adds an important layer of understanding, enabling more efficient and accurate remediation efforts.
As the world continues to grapple with the challenges of environmental pollution, biochar presents a sustainable and versatile solution. Its potential applications go far beyond chromium cleanup, offering a way to address a wide range of pollutants and contributing to cleaner, healthier ecosystems for future generations.
Reference: Filip Budimir et al, Chromium isotope fractionation during the removal of hexavalent chromium by oak-based biochar, Chemosphere (2024). DOI: 10.1016/j.chemosphere.2024.143880