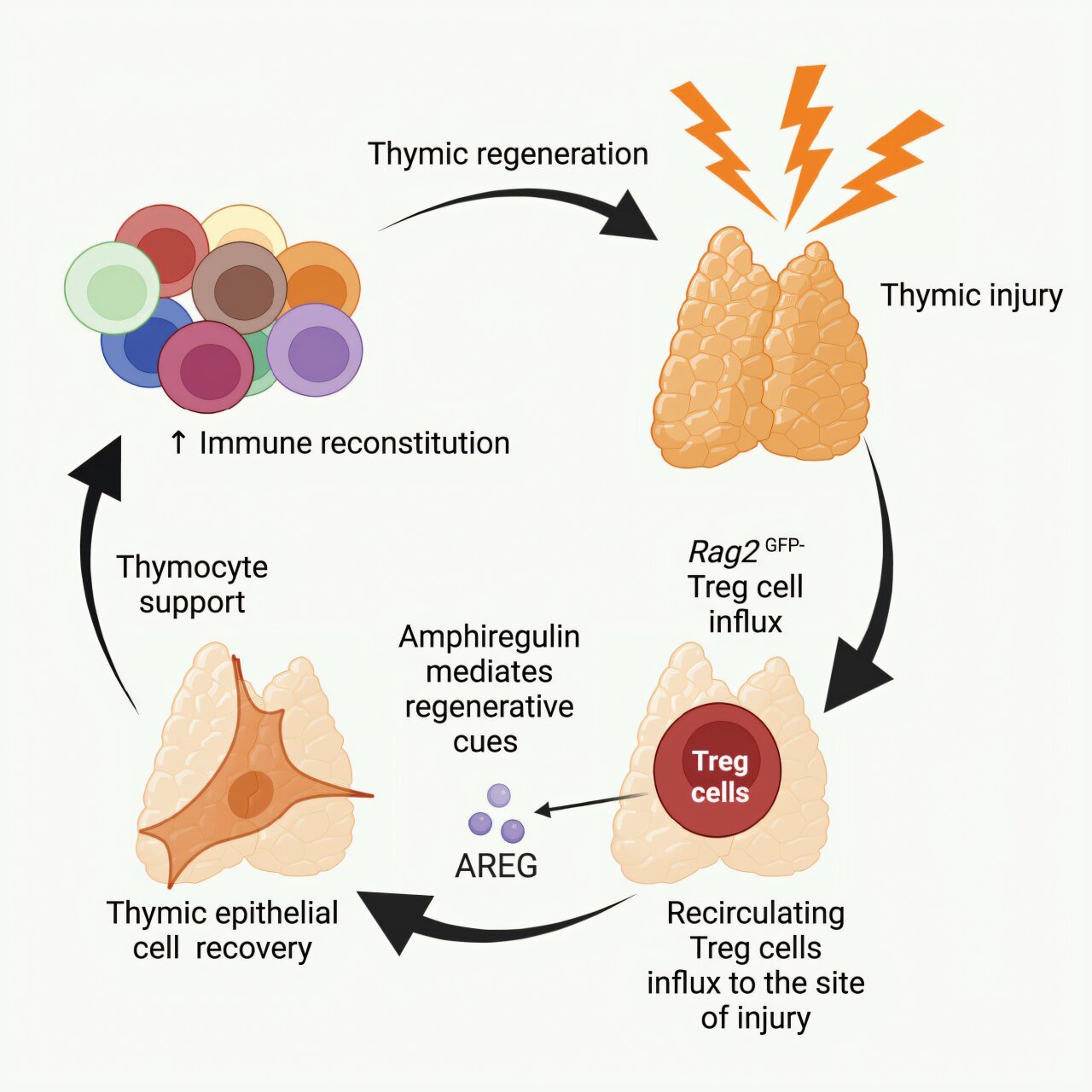
A team of international researchers, led by scientists at City of Hope, has unveiled a groundbreaking way to regenerate and repair the thymus gland after damage, a discovery that could significantly improve immune system recovery in cancer patients and the elderly. Their findings, published in Immunity, provide insight into a previously unknown mechanism in which a specific type of regulatory T cell (Treg) is able to home back to the thymus and stimulate its regeneration, offering potential solutions for individuals whose immune systems have been compromised due to cancer treatments, aging, or stress.
This discovery opens up new avenues for addressing thymic fatigue and the resulting immune reconstitution failure, conditions often faced by cancer patients undergoing aggressive treatments such as chemotherapy or radiation. The thymus, which plays a pivotal role in the development of immune cells, particularly T cells, can suffer damage from these therapies, leading to a weakened immune response and increased susceptibility to infections.
The Vital Role of the Thymus
Located between the lungs and in front of the heart, the thymus is a small but highly significant organ responsible for producing T cells, which are essential for the body’s immune defenses. These T cells help protect the body from harmful invaders such as viruses, bacteria, and even cancer cells. Moreover, they play a crucial role in maintaining immune system balance by preventing autoimmune responses, where the immune system mistakenly attacks healthy cells.
As lead researcher Dr. Andri Lemarquis explains, the thymus serves as a training ground for T cells, where they learn to differentiate between harmful and harmless entities. “Because there are so many different threats out there, we need an incredible diversity of these soldiers to defend us,” Dr. Lemarquis stated. “But we also need some kind of control, so we don’t get a civil war in our body, like an autoimmune disease. This requires a school to teach these cells to protect us, and that school is the thymus.”
However, despite its crucial role in immune function, the thymus is particularly vulnerable to damage. The damage can occur due to aging, stress, infections, and the cancer therapies that are often used to treat patients. As Dr. Lemarquis points out, “If you receive cancer therapies, your thymus is going to shrink. Without some help to grow again, patients won’t get what’s called immune reconstitution, in which new protective T cells are produced.” Without the regeneration of the thymus, patients become increasingly vulnerable to infections and other diseases.
Discovering the Key to Thymic Regeneration
The research team set out to explore the mechanisms behind thymus regeneration in two key scenarios: after cancer therapies and as a result of aging. Both cancer patients and the elderly face similar challenges—compromised immune systems that make them more susceptible to infections, cancer recurrence, and autoimmunity.
In the laboratory, the team first investigated how the thymus was damaged in murine models (mice), focusing on the conditions under which the organ might be able to recover. They combined imaging and machine learning techniques to track thymus regeneration and identify the key pathways that were activated during the healing process.
What they discovered was remarkable: a previously undiscovered subset of thymic regulatory T cells (Tregs) that accumulate in the thymus following injury. These cells secrete a growth factor known as amphiregulin, which plays a crucial role in stimulating the regeneration of the thymus. Through experiments, the researchers showed that when these Tregs were introduced into the bloodstream of mice via intravenous injection, they naturally returned to the thymus, where amphiregulin helped to promote the development of new T cells and support immune function.

Incredibly, the researchers were able to replicate these results in human tissue samples, confirming that the same mechanisms operated in humans as in mice. Dr. Lemarquis noted, “We were able to go from mechanistic insights in mice to seeing that the same pathways were operating in the human setting.”
A Potential Game-Changer for Immune Health
The findings from the City of Hope team are highly promising because they suggest a novel and therapeutic pathway to restore thymic function in patients who have suffered damage to their thymus. Dr. Lemarquis emphasized that Treg-based therapies could offer a potential solution to regenerate tissues and promote immune reconstitution in cancer patients and the elderly, for whom thymic function may be severely compromised.
“What we could show was that when we transferred Tregs into aged mice receiving cancer therapies, we could even boost their function,” Dr. Lemarquis explained. This was especially surprising because it was once believed that thymic function could not be restored in aging animals. However, this new research challenges that notion, revealing that older thymi are still receptive to regeneration when exposed to the right signals—such as those provided by the Tregs.
The results of this research suggest that Treg-based therapies could be used not only to boost immune function in young cancer patients but also in the elderly, who often experience thymic atrophy and immune decline as part of the aging process.
From Basic Science to Clinical Application
The work presented in the Immunity paper is a continuation of a research program that began in the late 1990s, led by Dr. Guido van den Brink, who focused on boosting immune reconstitution in cancer patients, particularly those undergoing bone marrow transplants. According to Dr. Lemarquis, “He laid the groundwork over decades that provided us with the models, tools, and data to get to where we are today.” Building on this foundation, Dr. Lemarquis and his team were able to merge their expertise in regulatory T cells with the goal of addressing thymic injury.
Currently, the researchers are expanding their work by analyzing a large atlas of human thymic samples taken from cancer patients at various stages of thymic regeneration. They are working to identify additional pathways that could be used to further enhance thymic recovery. This research may eventually lead to more effective treatments for patients whose immune systems are compromised due to cancer therapies, aging, or other factors.
Next Steps and Future Directions
Looking ahead, Dr. Lemarquis and his team are exploring ways to apply their findings in clinical settings. They are investigating the possibility of using synthetic biology to modify Tregs to overproduce amphiregulin or other beneficial factors that could help boost thymic regeneration. The ultimate goal is to develop a therapeutic approach that can restore thymic function in cancer patients, elderly individuals, or anyone whose immune system has been weakened by disease or treatment.
“We have a thymic research program now at City of Hope, with computational biologists and postdoctoral scholars working on different projects,” said Dr. Lemarquis. “I think there is great potential for Tregs and amphiregulin to be used therapeutically in patients receiving cancer therapies to ameliorate immune function.”
A Collaborative Effort
The study “Recirculating regulatory T cells mediate thymic regeneration through amphiregulin following thymic damage” was a collaborative effort between researchers at City of Hope, Memorial Sloan Kettering Cancer Center, University of Gothenburg in Sweden, Bambino Gesù Children’s Hospital in Rome, New York-Presbyterian Morgan Stanley Children’s Hospital, University of Washington, Fred Hutchinson Cancer Center in Seattle, and Weill Cornell Medical College in New York. This international collaboration underscores the significance of the discovery and its potential to transform immune system recovery for many patients.
Conclusion
The discovery of a specific population of regulatory T cells that can aid in the regeneration of the thymus after damage represents a breakthrough in immunology. This novel mechanism holds great promise for improving the immune system’s ability to fight infections and diseases, particularly in cancer patients and the elderly. By harnessing the power of Tregs and amphiregulin, researchers may be able to develop targeted therapies that could restore thymic function, improving immune reconstitution and enhancing patient outcomes.
As researchers continue to explore the full potential of this discovery, it is clear that the work at City of Hope marks an exciting step forward in the battle to restore immune health in patients suffering from thymic damage.
Reference: Andri L. Lemarquis et al. Recirculating regulatory T cells mediate thymic regeneration through amphiregulin following damage, Immunity (2025). DOI: 10.1016/j.immuni.2025.01.006. www.cell.com/immunity/fulltext … 1074-7613(25)00031-7