Psoriasis is a chronic, inflammatory skin condition that affects millions of people worldwide. Known for causing red, scaly patches of skin, it can be both physically painful and emotionally distressing. At the core of this disease lies an overactive immune system, which, inappropriately, triggers skin inflammation. However, recent research has uncovered deeper insights into the molecular mechanisms driving psoriasis flare-ups, offering hope for more targeted treatments.
In a groundbreaking study conducted by researchers at Case Western Reserve University School of Medicine, the protein NF-kB c-Rel has been identified as a key player in intensifying psoriasis symptoms. When activated by signals from the immune system, c-Rel can exacerbate skin inflammation, which forms the hallmark of psoriasis. Understanding how this protein behaves could pave the way for novel therapies to alleviate the discomfort experienced by individuals suffering from this condition.
The Role of Immune Cells and c-Rel in Psoriasis
Published in the journal eBioMedicine, the study focused on the behavior of dendritic cells (DCs)—immune cells that are crucial in initiating and regulating immune responses. DCs are responsible for detecting pathogens and activating T-cells, which, in turn, regulate immune responses. In psoriasis, this immune system function goes awry, leading to an overactive immune response that triggers the inflammatory processes associated with the condition.
Researchers found that c-Rel plays a central role in this process by enhancing the inflammatory response. Specifically, they investigated how c-Rel responds to signals from Toll Like Receptor 7 (TLR7), a receptor involved in the body’s immune response to pathogens. TLR7 is known to be activated by viral infections, and in the case of psoriasis, it contributes to the inflammation that worsens the condition. By activating c-Rel, TLR7 amplifies the immune response, leading to the characteristic skin lesions seen in psoriasis patients.
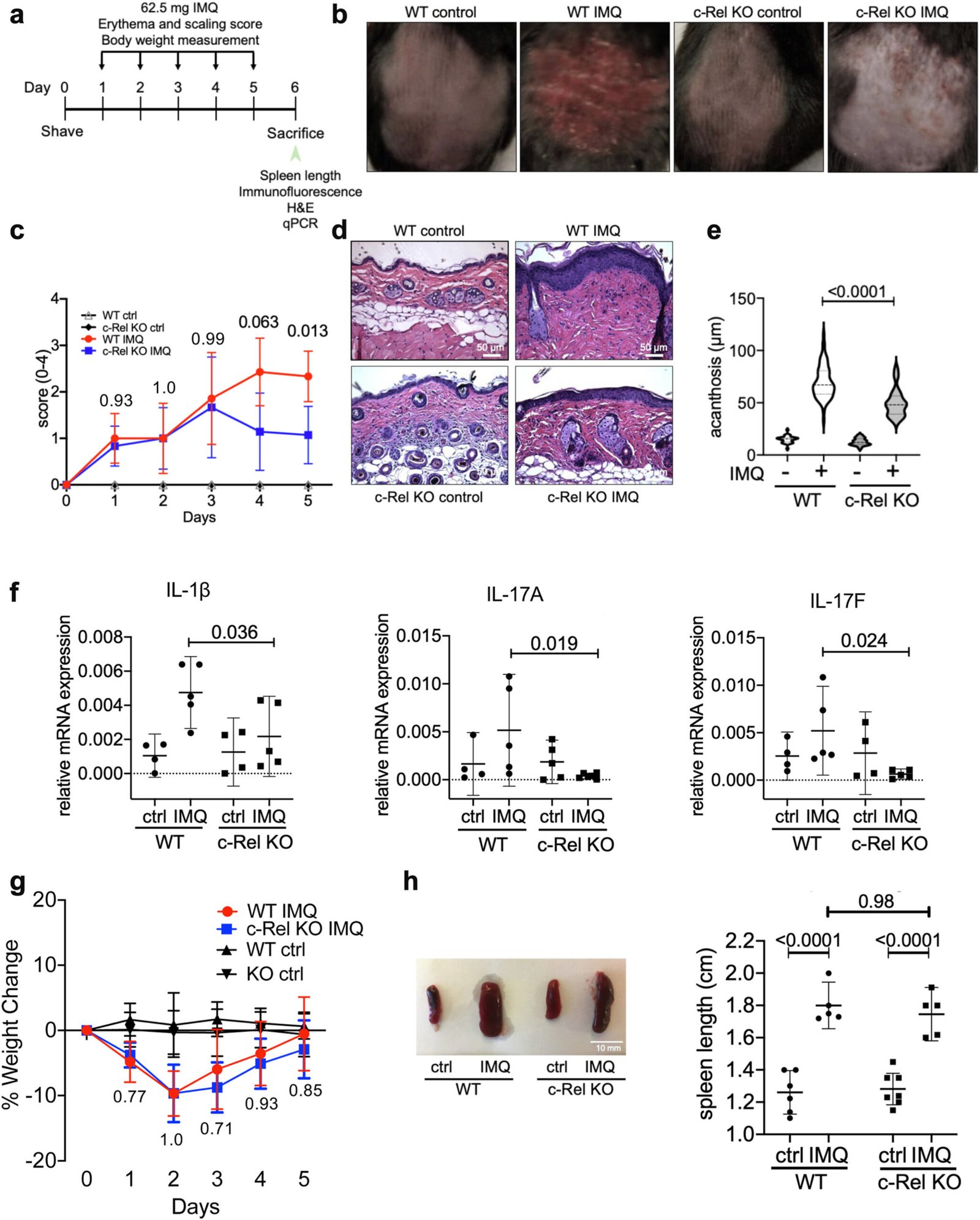
In their experiments, the researchers examined both human skin samples from psoriasis patients and a mouse model that mimics the skin changes associated with psoriasis. They analyzed the levels of c-Rel and its behavior in engineered cells that lacked the protein. Additionally, they studied a mouse model genetically modified to lack c-Rel, allowing them to observe the impact of its absence on inflammation.
Key Findings and Implications for Treatment
The study yielded significant findings, shedding light on the importance of c-Rel in psoriasis inflammation. First, they observed higher levels of c-Rel in the skin of individuals with psoriasis, confirming its role in amplifying the condition’s symptoms. In contrast, mice that lacked c-Rel were significantly protected from developing psoriasis, exhibiting notably less inflammation and fewer skin lesions. This suggests that targeting c-Rel could be a promising strategy for alleviating the symptoms of psoriasis.
Parameswaran Ramakrishnan, the study’s principal investigator, emphasized the potential for c-Rel and TLR7-targeted therapies in the future. “By focusing on c-Rel and TLR7, scientists might be able to create more targeted treatments that reduce inflammation and help psoriasis symptoms,” he said. This line of research could lead to precision medicine approaches that specifically target the molecular pathways responsible for psoriasis, reducing the need for generalized treatments with potential side effects.
The researchers also highlighted the connection between viral infections and psoriasis flare-ups, particularly those caused by viruses that activate TLR7, such as human immunodeficiency virus (HIV), human papillomavirus (HPV), and hepatitis C virus (HCV). These viruses are known to trigger immune responses that can worsen psoriasis, adding another layer of complexity to the disease. The findings from this study could lead to better understanding how viral TLR7 activation contributes to psoriasis flare-ups, offering new avenues for treatment in patients with both viral infections and psoriasis.
Broader Implications for Other Diseases
While the primary focus of this study was on psoriasis, Ramakrishnan noted that the research opens doors to exploring the role of c-Rel and TLR7 in other diseases where these proteins play a significant role. For example, systemic lupus erythematosus (SLE), an autoimmune disease, and wound-healing in diabetes, are both areas where c-Rel and TLR7 signaling could provide valuable insights.
“The research warrants future studies on TLR7-c-Rel-dependent molecular mechanisms regulating DC function,” Ramakrishnan added. “This could be a potential link for how viral TLR7 activation is involved in worsening psoriatic disease, and may even provide clues for other diseases with similar immune mechanisms.”
Future Directions and the Potential for Targeted Treatments
The research conducted by the team at Case Western Reserve University School of Medicine underscores the critical need for further studies into the molecular mechanisms that govern psoriasis. Understanding how proteins like c-Rel and TLR7 contribute to immune system dysfunction is a crucial step toward developing more effective, personalized therapies for psoriasis patients. These therapies could not only improve quality of life by reducing inflammation but also help mitigate the physical and psychological burden associated with the disease.
As the scientific community continues to explore the intricate relationship between the immune system and inflammatory skin diseases, the hope is that new treatments will emerge—ones that are more precise and targeted than the traditional therapies currently available. For the millions of people who live with psoriasis, these advancements could offer much-needed relief from the pain, discomfort, and social stigma often associated with the condition.
Conclusion
Psoriasis is a complex and challenging condition, but with the recent findings from Case Western Reserve University, the future looks promising for more effective treatments. By focusing on proteins like c-Rel and receptors like TLR7, researchers are opening up new possibilities for targeted therapies that could alleviate the inflammation at the heart of psoriasis. While much work remains to be done, the discovery of the pivotal role of c-Rel in psoriasis is a crucial step forward in the quest to understand and treat this debilitating disease.
With continued research, patients could soon have access to therapies that offer better symptom management and, ultimately, a better quality of life. The path forward is one of hope, driven by scientific discovery and the relentless pursuit of improved care for those affected by psoriasis and other inflammatory diseases.
Reference: Angela Rose Liu et al, NF-κB c-Rel is a critical regulator of TLR7-induced inflammation in psoriasis, eBioMedicine (2024). DOI: 10.1016/j.ebiom.2024.105452