Scientists at the University of California San Diego School of Medicine have made significant progress in understanding the complex factors that drive the development of liver cancer. This condition, the sixth most commonly diagnosed cancer and the fourth leading cause of cancer-related deaths globally, remains a major health challenge. Their findings, published in the prestigious journal Nature, uncover the intricate relationship between cellular metabolism, DNA damage, and the progression of fatty liver disease to hepatocellular carcinoma (HCC). These insights have profound implications for liver cancer prevention, treatment, and the broader understanding of how diet influences genetic stability.
Over the past two decades, the incidence of HCC, the most prevalent form of liver cancer, has surged by 25-30%. Much of this alarming rise can be traced to the increasing prevalence of fatty liver disease, which now affects one in four American adults. Fatty liver disease occurs when fat accumulates in liver cells, often due to poor dietary habits. Approximately 20% of individuals with this condition develop metabolic dysfunction-associated steatohepatitis (MASH), a severe form of fatty liver disease. MASH dramatically raises the risk of liver damage and cancer. Yet, the precise cellular and molecular mechanisms driving the transition from MASH to liver cancer have remained elusive—until now.
Michael Karin, Ph.D., a Distinguished Professor in the Department of Pharmacology at UC San Diego, explained the gravity of this progression. “Going from fatty liver disease to MASH to liver cancer is a very common scenario, and the consequences can be deadly,” he stated. Individuals with MASH face two primary outcomes: liver destruction necessitating transplantation or progression to potentially fatal liver cancer. However, Karin emphasized that what happens at the subcellular level during this process has long been a mystery.
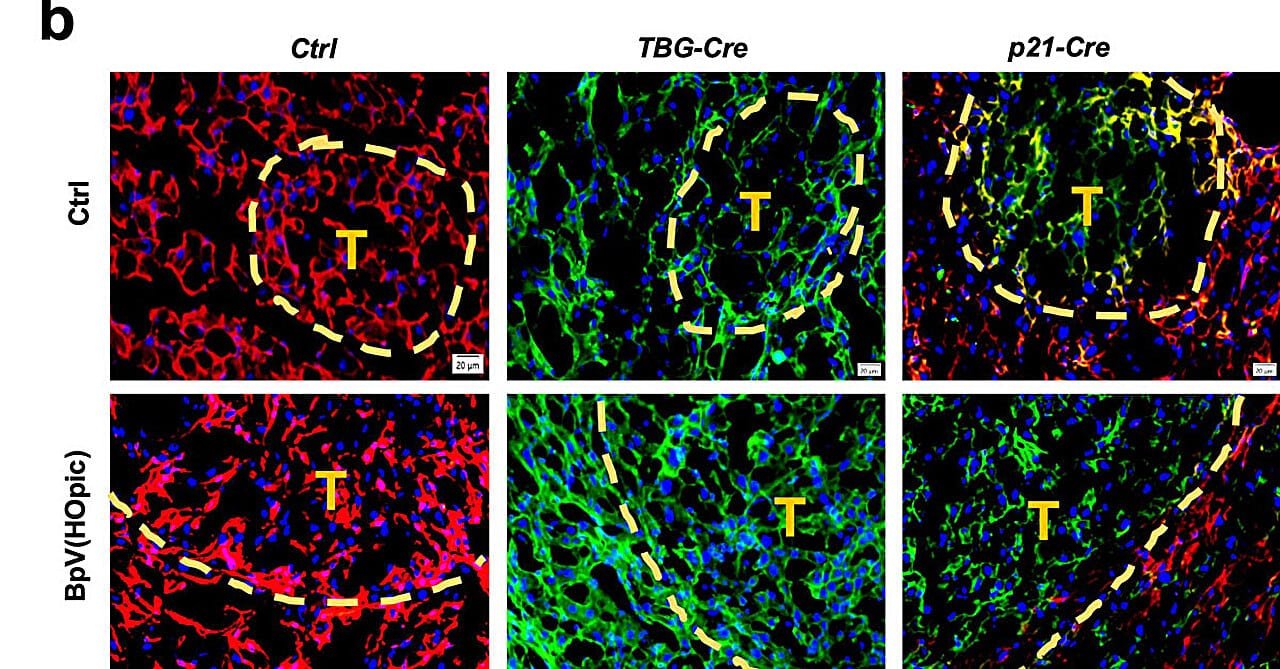
To unravel these complexities, the researchers employed a combination of advanced mouse models and analyses of human liver tissue samples and genomic databases. Their work focused on how diets high in fat and sugar—a major cause of MASH—trigger DNA damage in hepatocytes, the primary cells of the liver. This damage forces cells into a state of senescence. While senescent cells remain metabolically active, they lose their ability to divide, which typically serves as a protective mechanism to prevent damaged cells from turning cancerous.
However, hepatocytes respond differently. In some cases, damaged liver cells survive senescence and retain the capacity to re-enter the cell cycle. These “ticking time bombs,” as Karin describes them, pose a significant cancer risk because they can resume proliferation and eventually become malignant.
Ludmil Alexandrov, Ph.D., another key contributor to the study and an associate professor at UC San Diego, added, “Comprehensive genomic analyses of tumor DNA indicate that they originate from liver cells damaged by MASH, emphasizing a direct link between diet-induced DNA damage and the development of cancer.”
The implications of these findings are far-reaching. One major revelation is the critical role of DNA damage and repair mechanisms in the progression of liver cancer. This opens the door to the development of innovative therapeutic strategies aimed at preventing or reversing DNA damage in people with MASH. Karin and his team have proposed several potential avenues for future treatments.
One idea is the use of drugs or nutritional chemicals to address the imbalances in cellular components required for DNA synthesis and repair, which could be exacerbated by a high-fat diet. Another promising approach involves creating more efficient and targeted antioxidants that mitigate cellular stress and prevent DNA damage. While these strategies require further investigation, they offer a beacon of hope for reducing the growing burden of liver cancer.
Beyond its implications for liver cancer, the study also sheds light on the paradoxical role of cellular senescence in aging and cancer. Senescence is often viewed as a protective mechanism against cancer, but its association with aging suggests a more complex relationship. Aging is a major risk factor for almost all cancers, and senescent cells accumulate in aging tissues, increasing cancer susceptibility. Karin explained that this study provides crucial insights into the molecular mechanisms that enable senescent cells to re-enter the cell cycle and contribute to tumor formation. Understanding these processes could lead to broader applications in cancer research and therapy.
Equally important are the study’s revelations about the detrimental effects of poor diets on cellular metabolism and DNA integrity. Karin’s comparison of unhealthy dietary habits to cigarette smoking is particularly striking. While smoking has long been recognized as a major public health threat, this research underscores the significant, often underestimated dangers posed by a high-fat, sugar-laden diet. Such diets not only contribute to cosmetic and metabolic health issues but also fundamentally alter cellular processes, including DNA stability and repair.
These findings hold significant implications for public health policy and education. As the prevalence of fatty liver disease and related metabolic conditions continues to rise, there is an urgent need to raise awareness about the long-term consequences of poor dietary choices. Promoting healthier eating habits could play a crucial role in reducing the incidence of liver cancer and other chronic diseases associated with poor metabolic health.
Reference: Li Gu et al, FBP1 controls liver cancer evolution from senescent MASH hepatocytes, Nature (2025). DOI: 10.1038/s41586-024-08317-9