Researchers from the University of Pittsburgh have uncovered a novel and surprising connection between Alzheimer’s disease and herpes simplex virus-1 (HSV-1), highlighting a potential viral influence in the development of Alzheimer’s. The new findings, published in Cell Reports, suggest that the progression of Alzheimer’s could be influenced not just by the usual biological factors such as amyloid plaques and tau protein tangles, but also by viral infections that may trigger an immune response that can accelerate neurodegeneration. This research proposes a fundamentally different perspective on the role of tau protein, challenging the established view and potentially opening up new avenues for therapeutic treatments.
The study, led by Dr. Or Shemesh, an assistant professor at the University of Pittsburgh’s Department of Ophthalmology, suggests that tau protein—commonly known for its harmful role in Alzheimer’s disease, where it forms tangles inside brain cells—might serve as part of the brain’s initial defense mechanism against HSV-1 infection. This groundbreaking discovery points to the possibility that tau might initially have a protective role before eventually contributing to the very neurodegeneration seen in Alzheimer’s disease.
In normal circumstances, tau protein helps stabilize microtubules in the brain cells, particularly neurons, and ensures proper cellular function. However, in Alzheimer’s disease, tau proteins become abnormally modified and aggregate into tangles, which are considered one of the disease’s hallmarks. These tangles damage brain cells and hinder their ability to function correctly, leading to memory loss and cognitive decline. Until now, tau’s role has primarily been seen through this lens of harm.
However, according to Dr. Shemesh’s research, tau might not be entirely detrimental. The new research links HSV-1 to the activity of tau in the brain, specifically suggesting that tau protein could act as part of the brain’s immune response when the herpes virus infects neurons. HSV-1, a virus known for causing cold sores and other conditions, has long been linked to neurological diseases like encephalitis. This new connection proposes that HSV-1 may also influence the onset or progression of Alzheimer’s, especially considering that the virus remains dormant in the body and could be reactivated under certain conditions, including a weakened immune system.
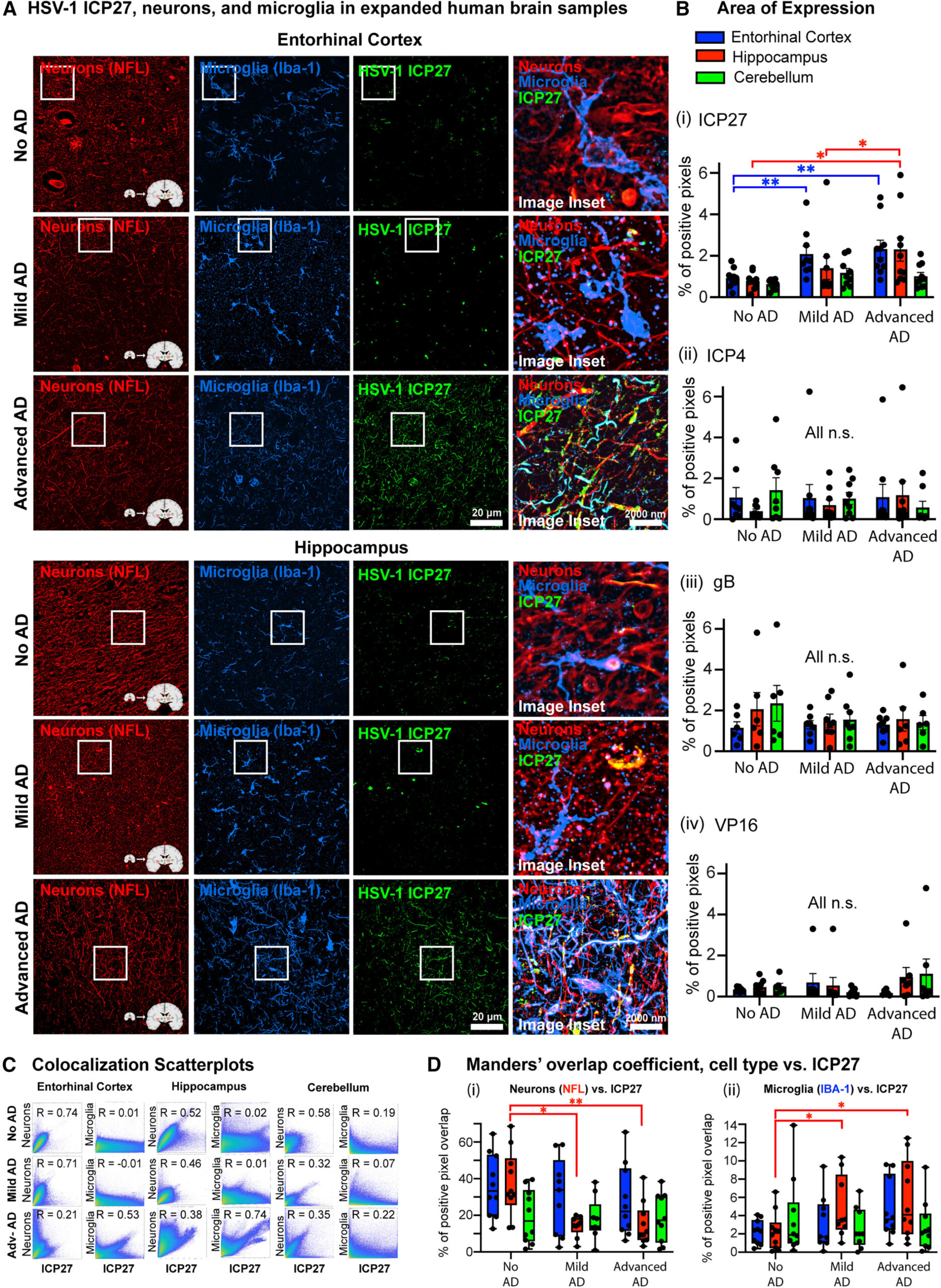
The research team analyzed post-mortem brain samples of Alzheimer’s patients and identified specific HSV-1-related proteins. The results revealed higher concentrations of viral proteins in brain regions typically affected by Alzheimer’s. Interestingly, these viral proteins were found in close proximity to tau tangles. The co-localization of these proteins with the phosphorylated tau tangles points to the possibility that HSV-1 might interact with tau in such a way as to modulate its normal functioning before it becomes harmful.
In laboratory settings, the team investigated miniature models of human brains grown in Petri dishes, simulating the infection of neurons with HSV-1. These studies suggested that HSV-1 infection could regulate the tau protein in the brain. Initially, tau’s modulation appeared to serve a protective role by preventing neuronal death following viral infection. This finding is intriguing because it suggests that the tau protein’s behavior may not always result in the neurodegeneration seen in Alzheimer’s, but might instead represent a form of compensation by the brain in response to infection. Over time, however, this protective effect might wear off, and tau protein could eventually contribute to long-term brain damage and disease progression.
Despite the significant insights into the role of HSV-1 in Alzheimer’s, the exact mechanisms through which the virus affects tau and neuronal function are not yet fully understood. Dr. Shemesh and his team plan further studies to explore how HSV-1 and tau proteins interact at the molecular level, with the aim of unraveling the precise pathways through which the virus may influence the brain. This knowledge could ultimately help in the development of new strategies to prevent or mitigate Alzheimer’s by targeting viral proteins or altering the immune responses that play a role in neurodegeneration.
The implications of this study extend beyond Alzheimer’s disease. Dr. Shemesh and his team also plan to investigate whether similar mechanisms are at play in other neurodegenerative diseases, including Parkinson’s disease and amyotrophic lateral sclerosis (ALS). As these diseases share certain common pathways of neurodegeneration, understanding whether HSV-1 or other viral infections contribute to their progression could provide valuable insights for treating a range of debilitating conditions.
The broader scientific community has long been interested in the role of infections in neurodegenerative diseases. Viruses, bacteria, and other pathogens are increasingly being recognized as potential factors in the development of diseases like Alzheimer’s, with mounting evidence suggesting that the immune system’s chronic activation in response to infections might play a significant role in accelerating neuronal damage. HSV-1, in particular, has been suspected of contributing to Alzheimer’s for some time due to its presence in the brains of many Alzheimer’s patients. However, direct proof of this connection has been sparse, and this research offers a significant step forward in understanding how a common viral infection may worsen or even trigger the disease in certain individuals.
The identification of tau protein’s role in immune defense adds a layer of complexity to our understanding of Alzheimer’s. Typically seen as part of the pathological process that contributes to memory loss and cognitive decline, tau might also serve as a double-edged sword. In the case of a viral infection like HSV-1, tau’s regulation of immune responses might initially mitigate some damage, but it might also contribute to cellular dysfunction as the infection persists or worsens. This complex interaction underlines the importance of a nuanced view of tau’s role in brain function.
From a therapeutic standpoint, the study’s findings offer an exciting new area of focus. For decades, the primary research into Alzheimer’s therapies has concentrated on removing amyloid plaques and inhibiting tau tangles. While these approaches have shown promise in some cases, they have not led to widespread cures or prevention strategies. A focus on viral infections and tau’s dual roles could present a new frontier in Alzheimer’s treatment, one that might aim not only to clear amyloid plaques but also to suppress or modulate viral infections in the brain and better regulate the immune response.
Furthermore, this research underscores the importance of an interdisciplinary approach to studying Alzheimer’s and other neurodegenerative diseases. Researchers from diverse fields, including virology, immunology, molecular biology, and neuroscience, will need to collaborate to fully explore the interactions between pathogens like HSV-1, the immune system, and brain proteins like tau. This research lays the groundwork for future drug development aimed at halting or slowing down the progress of Alzheimer’s and other neurodegenerative conditions linked to infections.
Reference: Vanesa R. Hyde et al. Anti-Herpetic Tau Preserves Neurons via the cGAS-STING-TBK1 Pathway in Alzheimer’s Disease, Cell Reports (2025). DOI: 10.1016/j.celrep.2024.115109
Think this is important? Spread the knowledge! Share now.