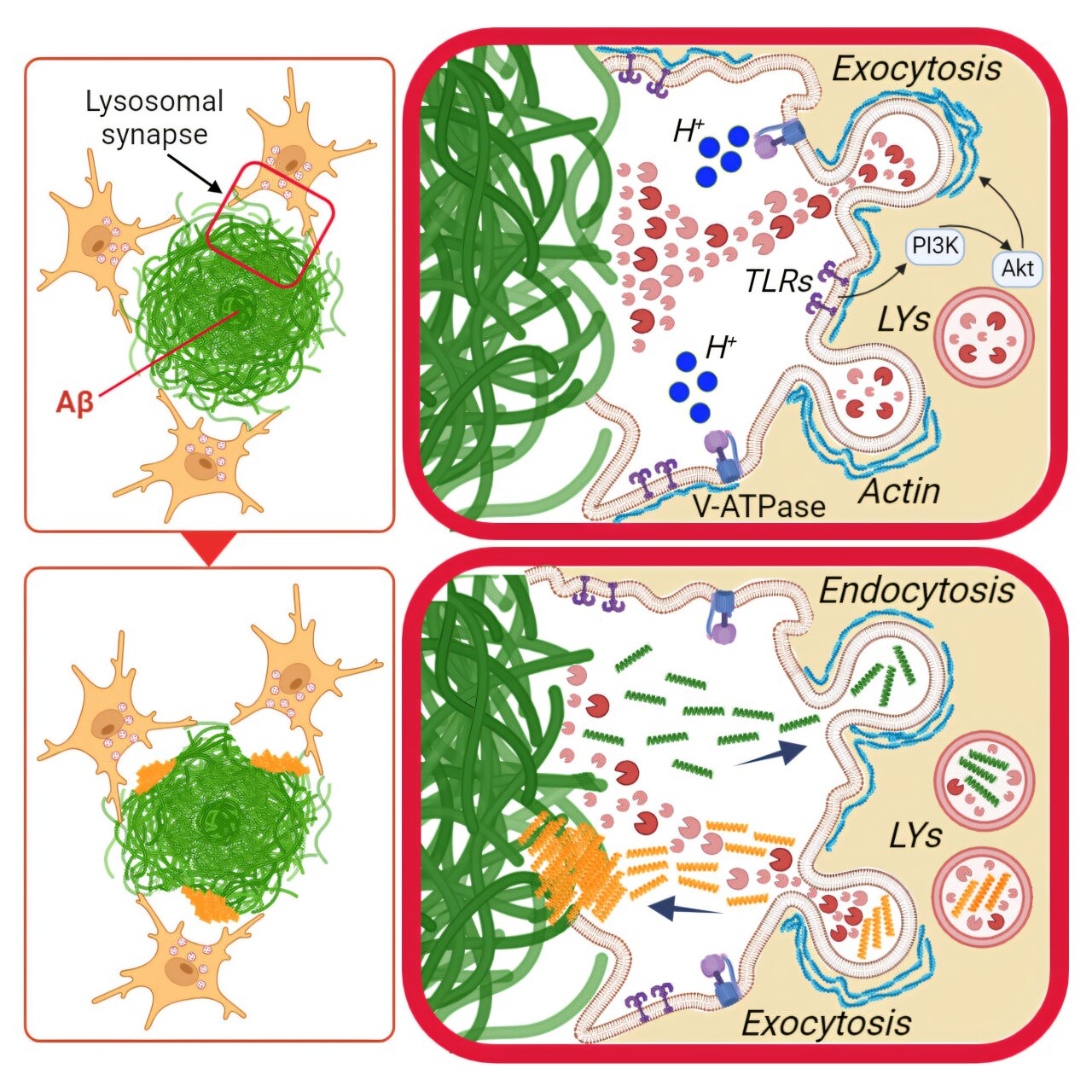
Recent studies have revealed that immune cells in the brain, known as microglia, can partially break down the large amyloid plaques associated with Alzheimer’s disease. By attaching themselves to these plaques, microglia form an external digestive mechanism to release enzymes that can digest the amyloid material. The breakthrough study, led by researchers at Weill Cornell Medicine, brings us closer to understanding the brain’s immune response in Alzheimer’s and could eventually provide therapeutic insights that boost microglia’s ability to remove these plaques. The research was published in Cell Reports and presents an intriguing new pathway called digestive exophagy, which may also provide answers to contradictory findings about how microglia might contribute to both plaque degradation and plaque spread in Alzheimer’s.
Microglia are the brain’s resident immune cells. Their primary function is to clear debris, dead cells, and pathogens from the brain, thereby maintaining a healthy neural environment. These cells constantly patrol the brain, using a process called phagocytosis, where they engulf and digest foreign matter or damaged tissue. Typically, microglia wrap around a small target, like a bit of cellular debris, and package it into vesicles. These vesicles are then transported to lysosomes, specialized compartments within the cell filled with enzymes designed to break down the ingested material.
Amyloid plaques, which are made up of aggregates of amyloid beta protein, are a hallmark of Alzheimer’s disease. These plaques can accumulate around neurons and disrupt their function, which is linked to cognitive decline. For years, scientists have understood that microglia play an important role in maintaining homeostasis in the brain, yet their involvement with amyloid plaques remained poorly understood, especially because plaques are far larger than the typical debris microglia consume. The mystery lay in how microglia could possibly digest something much larger than themselves.
The new research provides a compelling explanation for how microglia tackle these larger plaques. The team led by Dr. Frederick Maxfield and his colleagues demonstrated that when microglia encounter a large amyloid plaque, they attach a lysosome directly to the plaque’s surface. This lysosomal enzyme system then expels digestive enzymes into the surrounding space, breaking down the amyloid aggregates. This process, referred to as digestive exophagy, involves creating a partially sealed region around the plaque, mimicking the action of macrophages—a type of immune cell found throughout the rest of the body. Macrophages, as studied previously by the same team, are known to break down large clumps of substances like lipoproteins, and Maxfield theorized that microglia could use a similar approach for plaques.
The researchers validated this hypothesis in experiments with cultured mouse microglial cells. When placed near amyloid plaques in the lab, the microglia demonstrated the formation of an external vesicle around the plaque. The microglial lysosomes expelled their digestive enzymes into this space, helping to acidify it and activate the enzymes needed for amyloid breakdown. These observations were further confirmed by analyzing mouse models of Alzheimer’s disease. Co-author Dr. Marie-Ève Tremblay, using electron microscopy, imaged the mouse brain and found distinct pockets around the plaques, indicative of digestive exophagy. Enzymes involved in digestion were also detected within these pockets, supporting the team’s theory that microglia were actively engaging in this breakdown process.
Despite the clear evidence that microglia are breaking down amyloid plaques, their role in Alzheimer’s pathology is paradoxical. These same cells are also known to contribute to the spread of amyloid plaques across different regions of the brain. This suggests that while microglia can break down plaques, they might also play a role in their propagation. The study sought to explain this duality in microglial behavior. Previous research showed that when microglia were given small amyloid fibrils to consume, they took longer to degrade these particles in their internal lysosomes, and eventually, some fragments of amyloid were released back into the extracellular space. Could microglia be inadvertently spreading these fragments, contributing to plaque formation in other parts of the brain?
The new research offers a potential answer to this question. The researchers added amyloid fibrils to cultured microglia, and in a follow-up experiment, placed these pre-loaded cells near large amyloid plaques. They found that in these conditions, the microglia still performed digestive exophagy on the plaques, but the smaller fibril fragments they had already ingested were secreted towards the plaque, as the plaque was being broken down. The smaller pieces, now released back into the brain, could seed the formation of new plaques in nearby areas. This process could explain how amyloid plaques spread, as microglia are capable of moving to other locations in the brain after processing these debris fragments, thereby introducing seeds of amyloid in those regions.
These findings bring forth a complex narrative of microglial activity. While these cells can act as defenders, attempting to clean up the plaques, their mobilization might also contribute to the spread of Alzheimer’s pathology. Their movements and behaviors within the brain are far from simple scavenger actions; they are involved in processes that might lead to unintended consequences, such as seeding more plaque development.
The research also provides new avenues for possible therapeutic strategies. By boosting the ability of microglia to break down amyloid plaques, scientists may be able to halt or slow the progression of Alzheimer’s disease. Enhancing microglial function could prevent plaque accumulation or even promote the clearance of plaques already present in the brain. Understanding digestive exophagy could also be key in designing therapies that target and optimize this process.
An important next step in the research involves determining whether this digestive exophagy process also occurs in human cells. To study this, the team plans to use human induced pluripotent stem cells (iPSCs), which can differentiate into microglia and other types of brain cells. This will allow them to perform additional experiments and better understand if human microglia can also rely on digestive exophagy to clear amyloid plaques, and how this process may be manipulated for therapeutic purposes.
This research is an important milestone in the ongoing battle to understand and treat Alzheimer’s disease, as it not only uncovers new mechanisms at play in the brain’s immune response but also potentially reveals novel methods for therapeutic intervention. While more research is needed, these insights into the behavior of microglia provide promising leads that could pave the way for more effective treatments for Alzheimer’s and perhaps other neurodegenerative diseases marked by the buildup of toxic proteins.
Reference: Rudy G. Jacquet et al, Microglia degrade Alzheimer’s amyloid-beta deposits extracellularly via digestive exophagy, Cell Reports (2024). DOI: 10.1016/j.celrep.2024.115052