COVID-19, a disease primarily impacting the respiratory system, is caused by the SARS-CoV-2 virus, which was first detected in late 2019. As the pandemic unfolded, doctors and scientists began to observe an unexpected pattern in many COVID-19 patients: the disease wasn’t just affecting the lungs but was also inducing severe vascular complications. These complications, involving issues like blood clots, strokes, and heart attacks, seemed disproportionate to what would typically be associated with a respiratory infection. The SARS-CoV-2 virus was demonstrating an unusual capacity to cause widespread disruption in the body’s vascular system—an outcome that puzzled many health professionals.
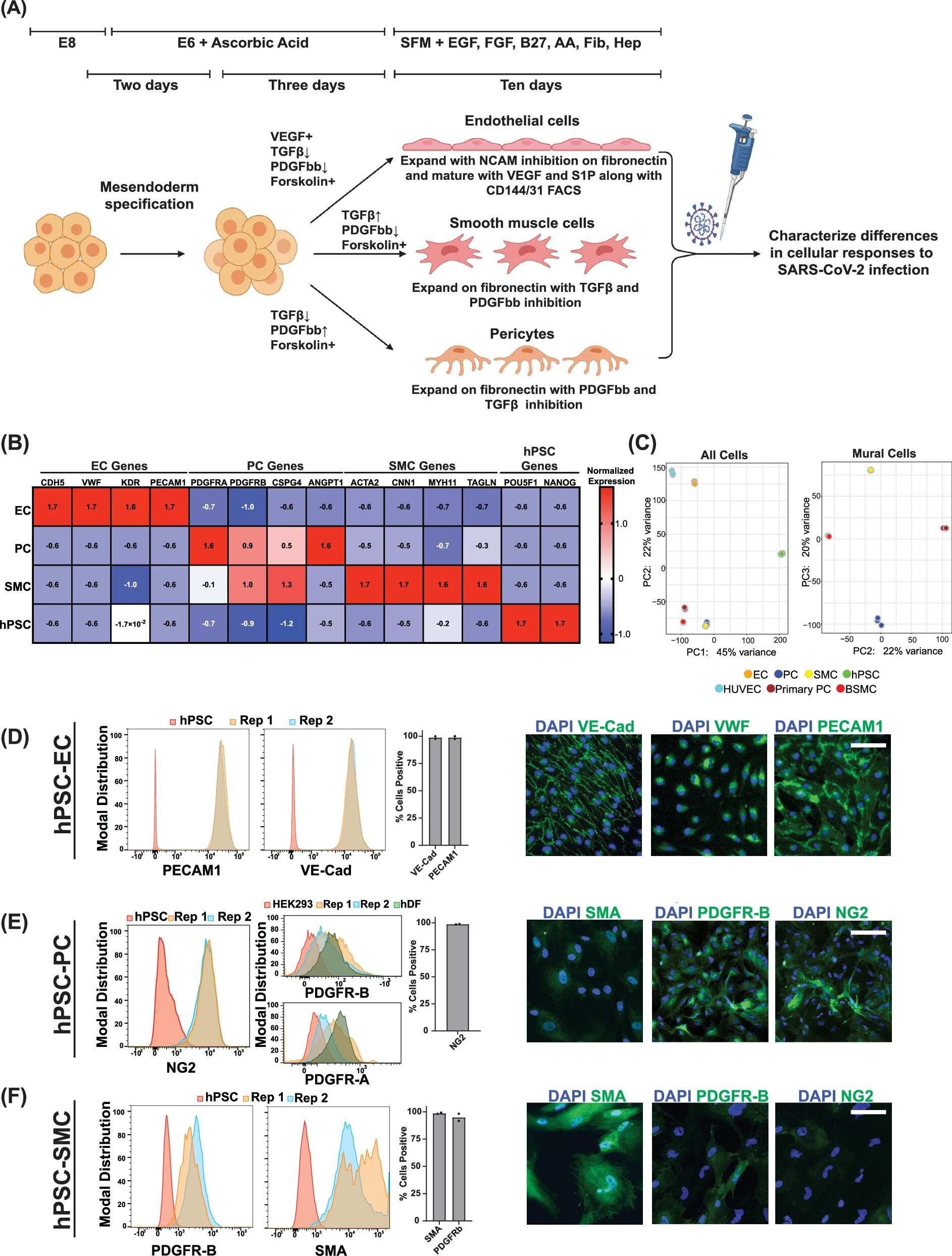
The vascular complications associated with COVID-19 intrigued a team led by Rudolf Jaenisch, a founding member of the Whitehead Institute. Jaenisch and his colleagues sought to investigate the virus’s impact on blood vessels and surrounding tissue in a detailed manner. To do so, they developed an innovative experimental model using pluripotent stem cells to generate relevant vascular and perivascular cell types. This allowed the researchers to study the effects of the virus on vascular cells in a controlled setting, which could provide insights into how the SARS-CoV-2 virus induces these serious and seemingly disconnected effects. These breakthroughs were published in Nature Communications, shedding light on a previously unexplored aspect of COVID-19’s pathology.
The study focused on three key cell types that contribute to vascular function: endothelial cells, smooth muscle cells, and pericytes, the perivascular cells that surround and support blood vessels. In traditional models, these cell types are often generated separately, leading to challenges in understanding their interactions during viral infection. By applying bioengineering techniques, the research team developed a new method for generating these three cell types together in a single culture. This approach proved vital for the next stage of their investigation.
Jaenisch’s team focused on understanding why vascular complications were manifesting in patients infected with the virus. To investigate how SARS-CoV-2 interacts with blood vessel cells, they first exposed their engineered system to the virus. Surprisingly, the virus did not infect endothelial cells, which form the lining of blood vessels, as directly as it did the smooth muscle cells and pericytes. The smooth muscle cells, in particular, showed a high degree of viral susceptibility. Infected smooth muscle cells activated robust inflammatory signaling pathways and released signals that affected surrounding cells, even without the virus directly invading the endothelial cells. This was a critical discovery: the transmission of signals from infected smooth muscle cells to adjacent endothelial cells contributed significantly to vascular damage, resulting in clotting risks and inflammation that are hallmark features of severe COVID-19 cases.
Endothelial cells play a crucial role in regulating the flow of blood and preventing blood from leaking into the tissues. In their healthy state, endothelial cells are tightly connected, forming a barrier that ensures blood remains within blood vessels. When these cells are exposed to viral signaling, the barrier weakens, creating an environment where blood can leak and where the virus might spread. However, the significant structural changes in endothelial cells did not occur until they were exposed to signals from infected smooth muscle cells. This was an unexpected finding, as it showed that SARS-CoV-2’s vascular effects were not only a result of direct infection but also of indirect signaling between different types of cells within the vascular system.
The cell signaling that occurred as a result of the infection highlighted a new dimension of COVID-19’s pathology. The virus was causing inflammation that seemed to initiate changes in gene expression within endothelial cells, triggering activation of immune responses. Importantly, this chain of events led to the expression of several genes involved in blood coagulation processes—key players in the formation of blood clots. This explained the prevalence of thrombotic events, such as strokes and heart attacks, in severe COVID-19 cases. It also reinforced the idea that SARS-CoV-2 was not only a respiratory virus but one that triggered systemic effects that could severely impact vascular health.
The insights gained from this stem cell-based model were incredibly timely. Researchers found that a later strain of SARS-CoV-2, the Omicron variant, had notably weaker effects on vascular cells compared to the original strain. This was consistent with clinical data showing fewer vascular complications associated with Omicron infections. While these findings do not mean the threat of vascular complications is entirely gone, they do provide critical information about how new variants may behave and how the body’s vascular system might respond differently to evolving strains of the virus.
In addition to uncovering the virus’s specific impact on vascular cells, Jaenisch and his colleagues were able to explore potential therapeutic approaches using their model. One promising therapeutic approach was the use of a drug called N,N-Dimethyl-D-erythro-sphingosine, which was shown to reduce infection of smooth muscle cells by SARS-CoV-2. This drug did not harm endothelial cells, thus showcasing the potential of this model not only for understanding virus-cell interactions but also for screening future treatments aimed at reducing the vascular complications of COVID-19.
While the reduced vascular complications seen in recent SARS-CoV-2 strains lessen the urgency for immediate interventions targeting these pathways, the research team stresses the utility of their model for addressing future viral threats. The viral landscape is constantly evolving, with new strains of SARS-CoV-2 or other pathogens emerging that might present similar or even greater risks for vascular damage. This model system could serve as a critical tool for quickly identifying therapeutic candidates during emerging health crises.
Another significant aspect of the research was the interdisciplinary approach. The collaboration between virologists, bioengineers, and molecular biologists allowed for the development of a novel model system that accurately mimicked human vascular interactions. This interdisciplinary method—combining knowledge and techniques from several fields—was essential for uncovering how SARS-CoV-2 caused such unusual vascular issues. As the authors note, understanding how cells influence each other in the context of viral infections is an often-overlooked area of virology. Their innovative model not only advanced the understanding of COVID-19 but also introduced a new framework for studying vascular biology.
Furthermore, this work is valuable not just for understanding COVID-19, but also for future investigations into vascular diseases more generally. The model system can be adapted to study other viruses and pathogenic agents that impact the vascular system, thus broadening its potential uses. It may prove essential in future drug development programs that aim to address diseases that involve blood vessel dysfunction.
Reference: Alexsia Richards et al, SARS-CoV-2 infection of human pluripotent stem cell-derived vascular cells reveals smooth muscle cells as key mediators of vascular pathology during infection, Nature Communications (2024). DOI: 10.1038/s41467-024-54917-4