Researchers at the Children’s Hospital of Philadelphia (CHOP) and the Perelman School of Medicine at the University of Pennsylvania have introduced an innovative technology designed to enhance the identification of antigen-reactive T cells, a critical step in improving immune responses against cancer. This new technology, Aptamer-based T Lymphocyte Activity Screening and SEQuencing (ATLAS-seq), offers a novel method for screening T cell receptors (TCRs) that can potentially play a crucial role in developing more effective immunotherapies for cancer patients. The findings of this study were recently published in Nature Communications.
The primary goal of this research is to aid in the development of immunotherapy treatments that enable the body’s immune system to identify and attack cancer cells. Tumor cells are known to present unique proteins, referred to as tumor antigens, on their surfaces. Immunotherapies aim to train the immune system to recognize and target these antigens more accurately. T cell receptors (TCRs), which are located on the surface of T cells, are essential in identifying these antigens and activating the immune response.
In cancer immunotherapy, TCRs play a pivotal role in recognizing these tumor antigens. Upon recognizing antigen peptides presented by major histocompatibility complex (MHC) molecules, TCRs initiate a series of events that lead to T cell activation, enabling the immune system to kill cancer cells. However, traditional methods for identifying antigen-reactive TCRs are not always successful in pinpointing the most effective receptors—those capable of triggering potent immune responses that can more efficiently target and eliminate cancer cells.
The challenge has been the ability of conventional methods to overlook those TCRs that may exhibit stronger functional activities. This is where ATLAS-seq shines, as it addresses one of the key obstacles in identifying TCRs that might not only recognize tumor antigens but also actively contribute to attacking and killing target cancer cells. According to Dr. Lan Lin, senior author of the study and Assistant Professor in Pathology and Laboratory Medicine at CHOP and Penn Medicine, TCRs identified by ATLAS-seq generally outperform those identified by conventional screening technologies when it comes to their ability to kill target cells.
“We’ve found that the TCRs identified using ATLAS-seq tend to be significantly more effective in targeting and killing cancer cells than those isolated using conventional technologies,” Lin explained. “This marks a major advancement in TCR screening and allows for more efficient identification of antigen-reactive TCRs that exhibit higher functional activity.”
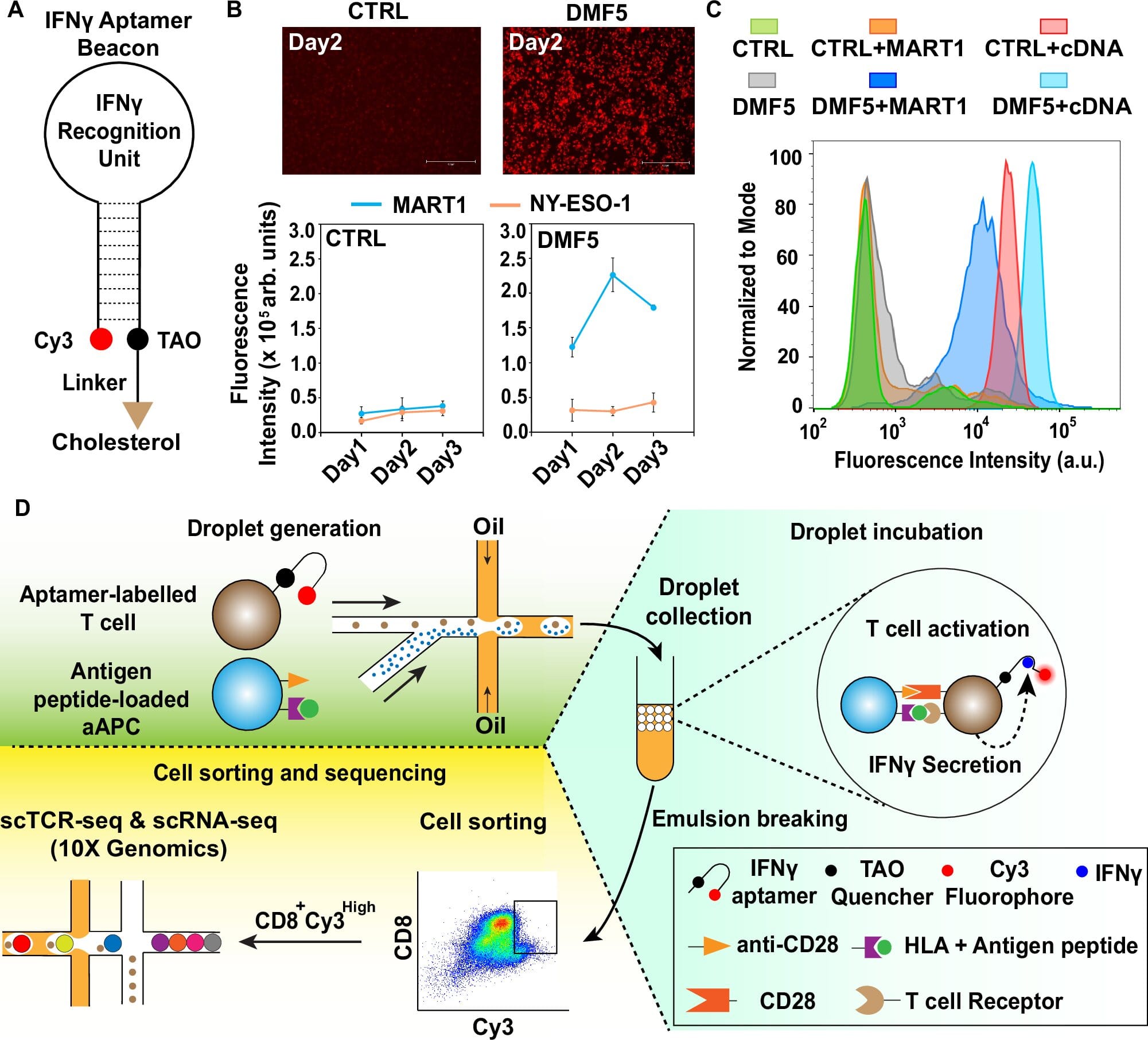
To achieve this breakthrough, the ATLAS-seq technology incorporates an advanced single-cell sequencing approach along with an aptamer-based fluorescent molecular sensor. This sensor is designed to emit a fluorescent signal when a T cell is activated, providing researchers with real-time insights into T cell activation. The study involved designing a microfluidic system capable of isolating individual T cells that respond to specific antigen peptides of interest. Once isolated, these T cells are sequenced at the single-cell level, allowing researchers to pinpoint their unique TCR sequences. This ability to perform single-cell sequencing ensures a higher resolution analysis compared to traditional methods, significantly enhancing the accuracy and reliability of TCR identification.
The combination of single-cell sequencing with aptamer-based fluorescence is particularly beneficial for a comprehensive analysis of T cell responses. TCR identification has historically been a challenging task, especially in the context of understanding how different TCRs contribute to varying levels of immune activity. The sensitivity of ATLAS-seq allows scientists to gain deeper insights into the functional behavior of individual T cells, distinguishing those with the greatest potential for generating strong, immune-activating responses against cancer cells.
The hope is that this technology will lead to more precise immunotherapies. By targeting tumor antigens more accurately, ATLAS-seq can potentially pave the way for the development of more effective treatments for hard-to-treat cancers. As Dr. Lin pointed out, “We envision that ATLAS-seq can play a pivotal role in identifying TCRs that specifically target tumor antigens, thereby accelerating the development of novel T cell-based therapies for various challenging cancers.”
This innovation is a significant step forward in the realm of cancer immunotherapy. Cancer remains one of the leading causes of death globally, and current treatments such as chemotherapy and radiation often fail to provide long-term cures and may cause substantial side effects. Immunotherapy has emerged as a promising alternative, particularly with the use of T cells modified to enhance their ability to target and destroy cancerous cells. Technologies like ATLAS-seq represent important advancements in the field, enabling researchers to hone in on more effective TCRs that could lead to more tailored, and thus more effective, cancer treatments.
ATLAS-seq is poised to not only revolutionize how researchers screen for antigen-reactive T cells but also serve as a foundation for accelerating the development of next-generation cancer immunotherapies. With continued research and optimization, these immunotherapies hold the potential to provide long-lasting, targeted solutions that can make a tangible difference in the lives of patients battling some of the most difficult and aggressive forms of cancer.
In addition to its potential for immunotherapy development, ATLAS-seq can also expand the scientific community’s understanding of the mechanisms behind immune responses to cancer. The ability to identify which TCRs are most successful in mounting an immune response against tumor antigens opens the door to valuable insights into immune resistance and tumor evasion strategies. This understanding could guide the design of therapies that not only boost the effectiveness of T cells but also overcome common obstacles like immune suppression within the tumor microenvironment.
The implications of this work extend beyond cancer alone. As immunotherapy continues to evolve, the ATLAS-seq technology may have broader applications across a variety of diseases. Autoimmune conditions, chronic infections, and even viral diseases could potentially benefit from this novel approach to identifying and understanding antigen-reactive TCRs.
As research into ATLAS-seq advances and the technology becomes more widely applied, the hope is to further optimize it for clinical use, leading to improved cancer treatments that are not only more effective but also more accessible to patients around the world. Ultimately, the ability to identify highly functional TCRs through innovative screening methods like ATLAS-seq has the potential to redefine cancer therapy and significantly improve patient outcomes on a global scale.
Reference: Siwei Luo et al, ATLAS-seq: a microfluidic single-cell TCR screen for antigen-reactive TCRs, Nature Communications (2025). DOI: 10.1038/s41467-024-54675-3
Think this is important? Spread the knowledge! Share now.