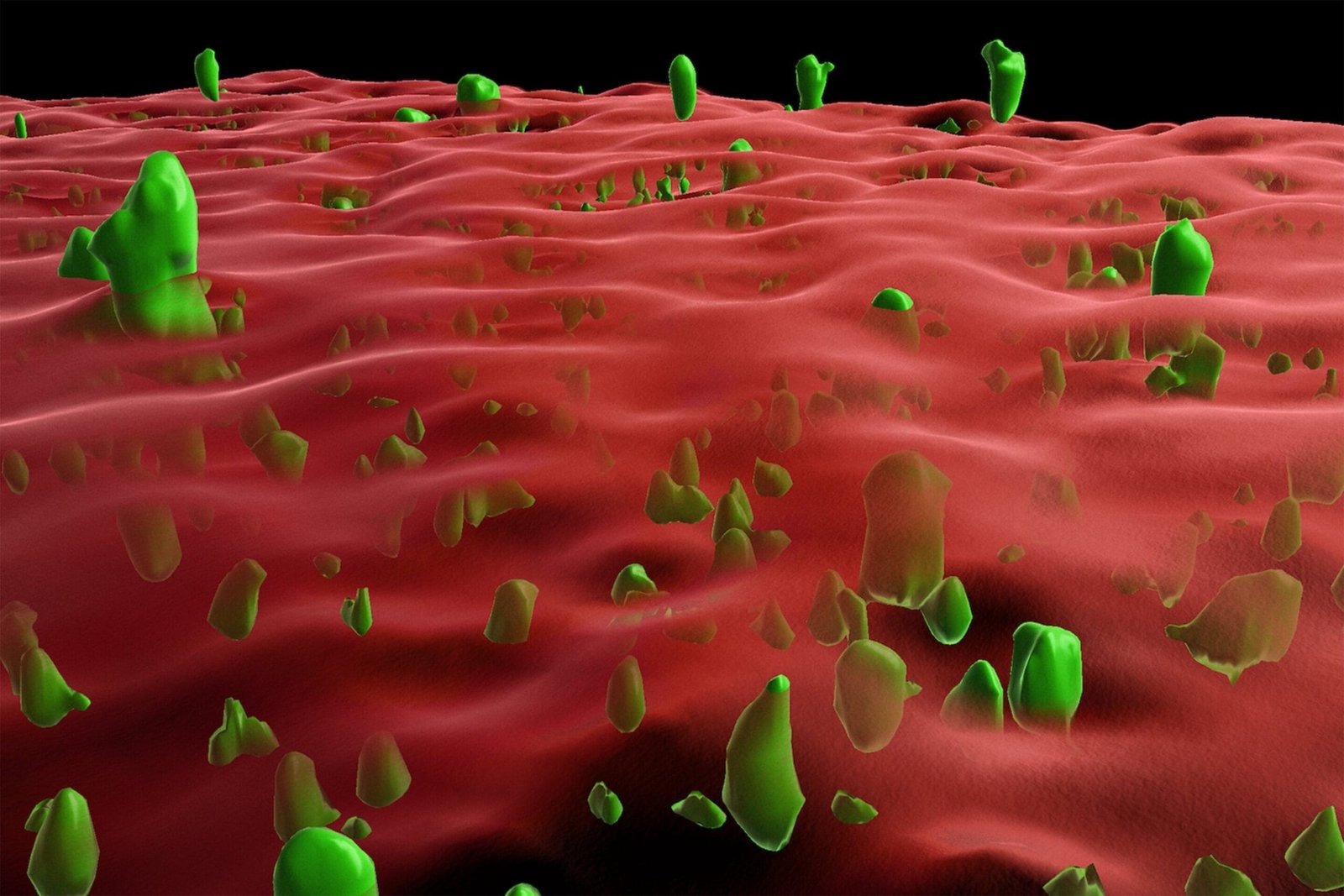
Protein aggregation is a hallmark of many neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and prion diseases such as Creutzfeldt-Jakob disease. These conditions are characterized by the accumulation of misfolded proteins in the brain, leading to cell damage, neuronal loss, and progressive cognitive and motor impairments. Unraveling the mechanisms behind this pathological transformation is crucial for developing effective therapeutic strategies. A recent study led by Professor Jörg Tatzelt from the Department of Biochemistry of Neurodegenerative Diseases at Ruhr University Bochum, Germany, sheds light on an intriguing aspect of protein aggregation inhibition. The researchers demonstrated that a lipid anchor on the outer membrane of nerve cells can significantly inhibit the aggregation of the prion protein.
This groundbreaking research, published in the Proceedings of the National Academy of Sciences on December 31, 2024, marks a step forward in understanding the molecular basis of prion diseases. According to Tatzelt, “Understanding the mechanisms that cause the originally folded proteins to transform into pathogenic forms is of crucial importance for the development of therapeutic strategies.” The findings from this study pave the way for novel approaches to tackle prion diseases and related neurodegenerative disorders.
Prion diseases are rare but invariably fatal brain diseases. They result from the misfolding of the prion protein (PrPC), a normal cellular protein, into its pathological form, known as scrapie prion protein (PrPSc). The misfolded PrPSc proteins aggregate into clumps, which disrupt neuronal function and cause the progressive degeneration of the brain. Prion diseases can be classified as hereditary, infectious, or sporadic. Hereditary prion diseases, caused by genetic mutations, are of particular interest due to their potential to reveal the underlying mechanisms of prion pathogenesis. Some mutations affect the attachment of PrPC to the cell membrane, yet the precise role of this membrane anchoring in disease progression remains poorly understood.
To address this knowledge gap, Tatzelt and his team developed innovative in vitro and cell culture models to investigate the influence of a lipid anchor on PrP folding and aggregation. The experiments revealed that membrane anchoring stabilizes the folding of PrPC, maintaining its functional conformation and inhibiting its transition into the disease-associated PrPSc form. The lipid anchor acts as a safeguard, reducing the likelihood of the protein forming aggregates that are characteristic of prion diseases.
One particularly compelling aspect of the study was the demonstration that pre-formed PrPSc aggregates could induce the aggregation of membrane-anchored PrPC. “What’s interesting is that the clumping of membrane-anchored PrP could be induced by pre-formed protein aggregates,” Tatzelt noted. This observation is significant because it mirrors the infectious nature of prion diseases, where misfolded proteins propagate their abnormal conformation to healthy proteins, leading to a chain reaction of aggregation.
The dual role of the lipid anchor in stabilizing PrPC and mediating its potential aggregation in the presence of PrPSc underscores its complexity in the context of prion diseases. This finding has far-reaching implications for understanding how prion diseases develop and spread. It also highlights the need for therapeutic strategies that preserve the stabilizing effects of membrane anchoring while preventing the interaction with pathogenic aggregates.
Beyond prion diseases, these insights may be relevant to other protein misfolding disorders such as Alzheimer’s and Parkinson’s diseases, which share common mechanisms of protein aggregation and neuronal damage. Understanding how cellular environments and molecular interactions influence protein folding and stability could inspire novel interventions to halt or reverse disease progression.
This study opens several avenues for future research. For instance, further investigation is needed to determine how different genetic mutations affecting membrane anchoring influence the folding and aggregation of PrPC. Moreover, exploring the biochemical and structural aspects of the lipid anchor could provide new targets for drug development. Additionally, the interplay between lipid composition, membrane dynamics, and protein folding merits detailed examination to fully elucidate their roles in neurodegenerative diseases.
The study also emphasizes the importance of developing advanced experimental models to mimic the complex environment of neuronal cells. By replicating the conditions under which protein aggregation occurs in the brain, researchers can gain deeper insights into the molecular triggers and modulators of these processes. These models could serve as valuable platforms for screening potential therapeutic compounds aimed at stabilizing protein folding or disrupting aggregation pathways.
Therapeutic strategies inspired by this research might include molecules that mimic or enhance the stabilizing effects of the lipid anchor, preventing PrPC from transitioning into its pathological form. Alternatively, approaches that inhibit the interaction between membrane-anchored PrPC and PrPSc could block the propagation of aggregation, potentially halting disease progression.
Reference: Kalpshree Gogte et al, Topological confinement by a membrane anchor suppresses phase separation into protein aggregates: Implications for prion diseases, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2415250121