In the same way a bear instinctively lowers its body temperature to survive the harsh winter cold, scientists have unlocked a fascinating discovery that might one day enable doctors to control human body temperature. This breakthrough could have significant life-saving implications, particularly in high-stress medical situations such as heart attacks, strokes, and other critical emergencies.
A team of researchers at Oregon Health & Science University (OHSU) led by Domenico Tupone, Ph.D., have found a method that might allow physicians to lower a patient’s body temperature in a controlled manner during life-threatening events, a process that mimics natural phenomena observed in certain animals during hibernation. For animals like bears or Arctic ground squirrels, the ability to enter a hibernation-like state—where the body temperature drops drastically and the metabolic rate slows—ensures survival through cold winter months when food is scarce and oxygen availability is low. Inspired by these natural processes, Tupone and his team may have just discovered a similar process that could be safely applied in humans.
The concept behind the discovery is to bring the body temperature down temporarily, reducing the demands for oxygen by vital organs like the brain and heart, in situations where oxygen supply is critically compromised. Whether it be due to stroke, heart attack, or prolonged surgery, ischemia (the lack of oxygen reaching tissues) can cause significant damage to cells, impairing organ function and recovery. By reducing the body temperature in a controlled manner, doctors could minimize the metabolic rate of tissues and slow down the deterioration, giving patients a better chance of surviving these types of life-threatening conditions.
“Reducing body temperature essentially buys more time for these tissues to survive without the necessary oxygen. This way, we might prevent further damage and improve the patient’s recovery after a stroke or heart attack,” explained Dr. Tupone, the senior author of the study and an expert in neurological surgery at OHSU’s School of Medicine. While humans cannot naturally hibernate like certain animals, the OHSU discovery may open up a new frontier in medical science where a similar effect can be achieved under controlled conditions.
In typical mammals, when the environmental temperature drops, the body’s thermoregulation system works to prevent the body from losing heat. The body tries to generate more warmth, either through shivering or through metabolizing brown adipose tissue, which is specialized fat designed to generate heat. The brain carefully orchestrates this process to keep the body temperature stable, allowing the mammal to survive in colder conditions.
However, hibernating animals like bears exhibit a strikingly different physiological response. As the temperatures fall, the mechanisms usually in place to generate heat are reprogrammed during their hibernation or “torpor” state. The body’s normal thermoregulation responses are flipped on their head: exposure to cold actually leads to the suppression of heat generation, allowing the animal to save energy and survive in conditions that would otherwise be fatal. For these animals, entering torpor is akin to going into a protective deep sleep, during which most metabolic processes, including those that require oxygen, slow down substantially.
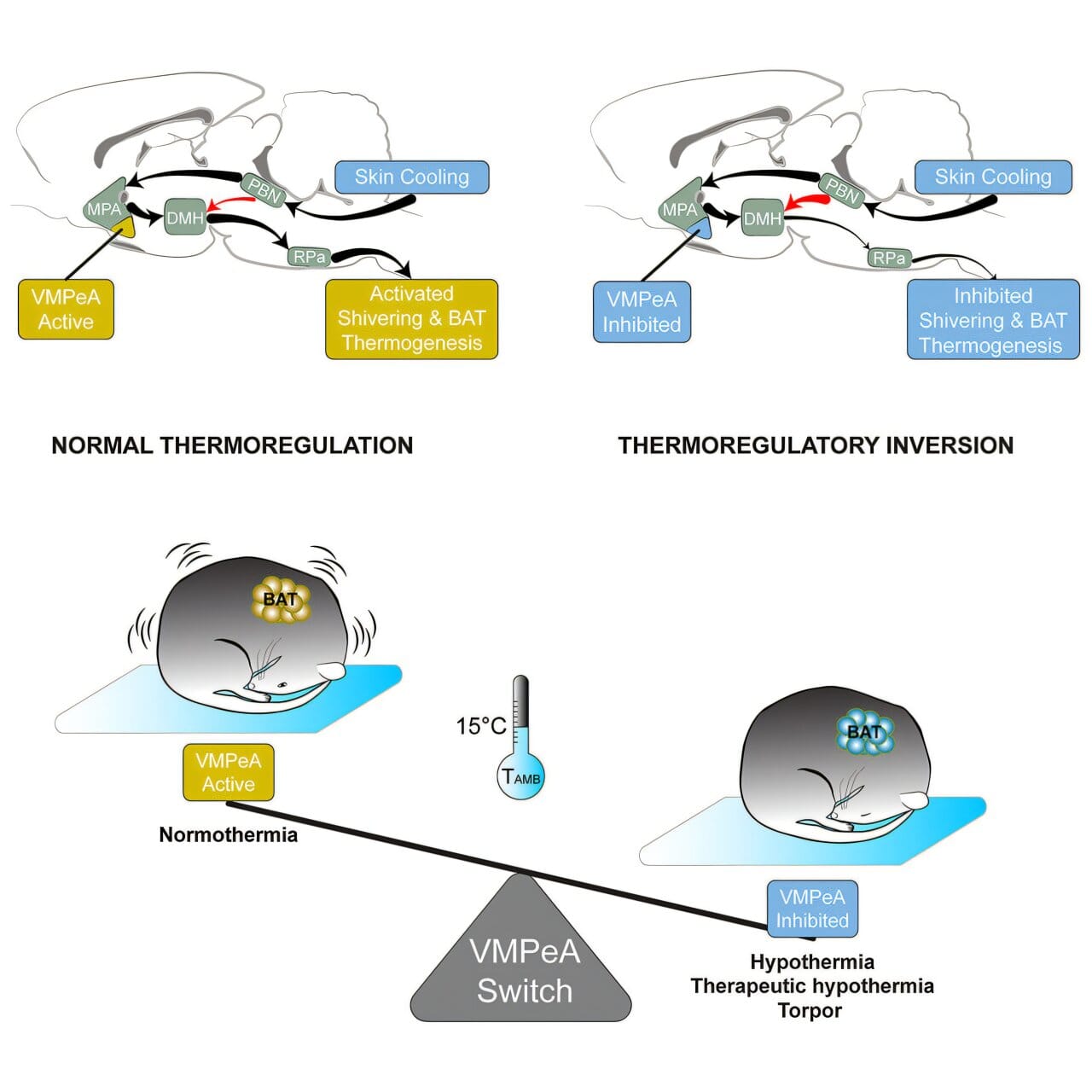
In their recent study published in Current Biology, Tupone and his colleagues revealed an astonishing finding: by blocking a specific area in the brain known as the ventromedial periventricular area (VMPeA), they were able to trigger this torpor-like state in rats, animals that do not naturally undergo hibernation. In essence, the VMPeA serves as what the researchers called a “torpor switch.” When this brain region is active, the rats’ bodies responded to temperature changes as one might expect—attempting to warm up in cold environments and cooling down when exposed to heat. However, by inhibiting the activity of the VMPeA, the rats’ natural thermoregulatory responses were turned off. Their bodies stopped producing heat in cold conditions and avoided heat production even when they were exposed to warmth, effectively lowering their body temperature to a state similar to hibernation.
This process—referred to as “thermoregulatory inversion” (TI)—could hold the key to inducing a state of controlled hypothermia in mammals that do not naturally hibernate, including humans. For physicians, this discovery might be the breakthrough that revolutionizes emergency medicine. Therapeutic hypothermia, which involves deliberately lowering the body temperature to slow metabolism and decrease oxygen demand, is already a common practice in the treatment of stroke patients and those who experience heart attacks. However, it is currently a challenge to safely manage this process in a precise and controlled way.
“If we had a mechanism that allows us to transform humans into hibernating animals, we could achieve and control therapeutic hypothermia much better,” said Dr. Tupone. “This opens up a new direction in medicine where we can use controlled hypothermia to protect organs and tissues during severe illnesses or interventions.”
The current approach to inducing hypothermia relies on cooling techniques, such as ice packs or special cooling devices, which can reduce the body’s temperature gradually. While effective, there are still challenges to ensure the process doesn’t harm organs or lead to complications like infections or blood clotting. With the new insights from this study, it may be possible to have more finely-tuned control over body temperature reduction, potentially giving doctors a much-needed tool to combat issues caused by ischemia or oxygen deprivation without the risks associated with traditional cooling methods.
Beyond treating stroke and heart attack patients, the findings from the study could have broader applications across various fields. For example, controlled hypothermia could improve outcomes for patients undergoing long and complex surgeries by reducing tissue damage that may occur due to the constraints of blood supply. Similarly, it could be a critical solution for managing traumatic brain injuries (TBI), where inducing a temporary, reversible drop in body temperature could protect the brain and help recovery.
Another intriguing possibility lies in long-term space travel. Scientists envision human explorers traveling to distant planets, and during such space missions, there may be times when crew members would need to enter a state of suspended metabolic activity to conserve resources, especially in situations where medical care is distant or unavailable. If controlled hypothermia can be perfected using this new method of manipulating brain activity, it could one day help sustain astronauts during extended voyages, reducing the oxygen and food requirements and protecting their bodies from the rigors of long-duration space travel.
By exploring how the brain can alter the normal responses to temperature regulation, Tupone’s research has opened the door to an entirely new domain of medical treatments. The discovery of thermoregulatory inversion represents a novel approach to controlling metabolism and protecting the body’s most vital organs when every second counts. While there are still significant hurdles to overcome before this process is safe and viable for humans, this groundbreaking work highlights an exciting new way to approach life-threatening conditions.
The potential of this research is only just beginning to be realized, but if further studies prove successful, controlled hypothermia using the brain’s natural mechanisms could one day become a standard medical technique. Not only could it save lives in emergency medical situations, but it may also offer a new direction in critical care and even space exploration, unlocking the possibility of inducing states that preserve life during some of humanity’s most challenging moments.
Reference: Shaun F. Morrison et al, Inhibition of the hypothalamic ventromedial periventricular area activates a dynorphin pathway-dependent thermoregulatory inversion in rats, Current Biology (2024). DOI: 10.1016/j.cub.2024.11.006