In a groundbreaking study, an international team of scientists from the United Kingdom and the United States has made a significant discovery about melanoma treatment, particularly in relation to immunotherapy. Melanoma, a type of skin cancer that originates in pigment-producing cells called melanocytes, is notorious for its aggressive nature and ability to spread quickly. While immunotherapy has emerged as a powerful treatment for various cancers, including melanoma, the reality is that it is only effective for about half of the patients. This disparity in response has made treatment selection a complex and often trial-and-error process. Patients who do not respond to immunotherapy may experience debilitating side effects without the desired therapeutic benefit, leading to potential worsening of their condition. The team’s discovery holds great promise in addressing this challenge by providing a more accurate way to predict which melanoma patients are likely to respond to immunotherapy, ultimately enabling clinicians to make more informed decisions about treatment options.
The study, which was published in JCO Oncology Advances, is the result of collaboration between the Universities of Bath (UK) and Stanford University (CA, U.S.), and it focuses on identifying biomarkers—specific biological indicators—that can predict the effectiveness of an immunotherapy treatment called TVEC (Talimogene laherparepvec). TVEC is a modified oncolytic virus that is injected directly into melanoma tumors with the aim of stimulating the immune system to recognize and attack the cancer cells. Oncolytic viruses like TVEC work by infecting and killing cancer cells, while simultaneously activating the immune system to target and destroy these cells. TVEC has been approved for use in advanced melanoma, but the current study marks the first time this treatment has been investigated for high-risk stage II melanoma patients.
Traditionally, it has been believed that TVEC works by primarily activating T cells, a type of white blood cell that plays a crucial role in the immune response against cancer. These T cells are thought to recognize and attack cancer cells, leading to tumor shrinkage. However, the findings from this study challenge this conventional understanding. Instead of T cell activity, the researchers discovered that the activity of macrophages, another type of white blood cell, could be used as a predictor of whether a patient would respond to TVEC treatment.
Macrophages are essential components of the immune system, responsible for engulfing and digesting pathogens, cancer cells, and other foreign material. They also play a crucial role in modulating the immune response, and their behavior can influence the success or failure of various treatments. In this study, the team found that changes in macrophage activity, both before and after TVEC treatment, were closely associated with which patients showed a positive response to the therapy. This discovery is significant because it suggests that the immune response in melanoma is more complex than simply measuring the presence of T cells. Instead, a more detailed understanding of the immune system as a whole, including macrophage activity, is crucial for predicting treatment outcomes.
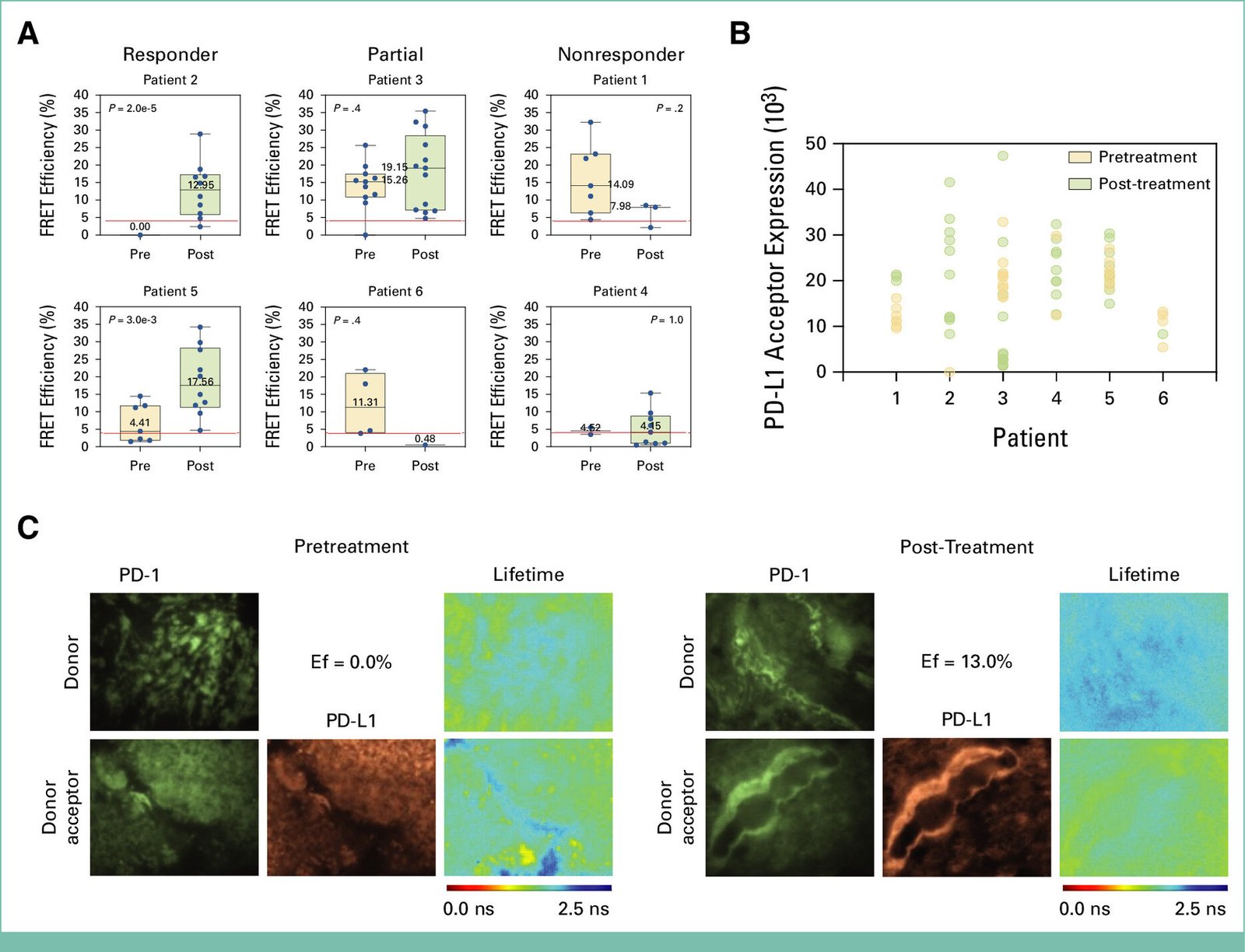
One of the key findings of the study was the use of a novel method called iFRET (Förster resonance energy transfer), which allows scientists to monitor protein activation rather than merely measuring the amount of protein present. Traditional methods have focused on measuring protein levels, such as the presence of PD-L1, a protein often used as a biomarker to assess immunotherapy effectiveness. However, this study showed that measuring the amount of PD-L1 or other proteins was not sufficient to predict whether a patient would respond to treatment. Instead, the researchers found that the activation of immune checkpoint regulators—proteins that help regulate the immune system—was a much more reliable indicator of response to therapy. Specifically, they observed a significant increase in macrophage infiltration after TVEC treatment in patients who responded positively. This infiltration was associated with high activation of immune checkpoint regulators, signaling an effective immune response against the tumor.
The implications of these findings are profound. The ability to predict whether a patient will respond to TVEC treatment—and potentially other immunotherapies—before the therapy even begins is a major step forward in personalized medicine. By identifying the specific biomarkers that correlate with treatment response, clinicians can select the most appropriate treatment for each patient, potentially saving time, minimizing side effects, and avoiding the use of ineffective therapies. This approach moves away from the traditional trial-and-error method and towards a more targeted and evidence-based treatment strategy.
Professor Banafshé Larijani, co-lead of the study and Director of the Centre for Therapeutic Innovation at the University of Bath, emphasized the importance of this finding. She noted that not all patients respond to immunotherapy in the same way—while some experience significant tumor shrinkage, others do not respond at all. The study shows that it is not enough to only consider T cell activity when assessing a patient’s potential response to immunotherapy. Instead, the researchers argue that it is crucial to examine the entire immune response environment in detail, including macrophage activity. In non-responding patients, the team suggests that targeting these macrophages could help reprogram the tumor immune environment, potentially enhancing the effectiveness of the treatment.
Dr. Amanda Kirane, Director of Cutaneous Surgical Oncology at Stanford University School of Medicine, who led the clinical aspect of the study, also highlighted the significance of the research. She explained that the study established a connection between pre-existing innate immune functions and the ability to respond to immune-stimulating drugs like TVEC. The study further supports the growing evidence that there are biological differences between patients who are likely to respond to oncolytic viruses like TVEC and those who may respond better to other types of immunotherapies that target immune checkpoint regulators. Additionally, the study sheds light on the limitations of measuring protein levels like PD-L1 as a clinical biomarker. The use of iFRET-based immune activity measurements, which focus on protein activation rather than just protein presence, could provide critical insights into why current biomarkers have failed to predict treatment outcomes reliably.
Moving forward, the research team plans to delve deeper into the cellular mechanisms involved in immune checkpoint interactions. By gaining a more comprehensive understanding of the cells that contribute to immune checkpoint activation, the team aims to improve patient stratification, which would further enhance the tailoring of personalized medicine. This approach would help ensure that patients receive the most effective treatments based on their unique immune profiles.
Reference: Amanda R. Kirane et al, Toward Functional Biomarkers of Response to Neoadjuvant Oncolytic Virus in Stage II Melanoma: Immune-Förster Resonance Energy Transfer and the Dynamic Tumor Immune Microenvironment, JCO Oncology Advances (2025). DOI: 10.1200/OA-24-00049
Think this is important? Spread the knowledge! Share now.