Researchers at University College Dublin (UCD) have made a significant breakthrough in the study of inherited retinal diseases, uncovering a crucial genetic mutation that leads to severe vision problems in zebrafish. This discovery holds promise for understanding similar diseases in humans and potentially offering new therapeutic approaches for inherited blindness.
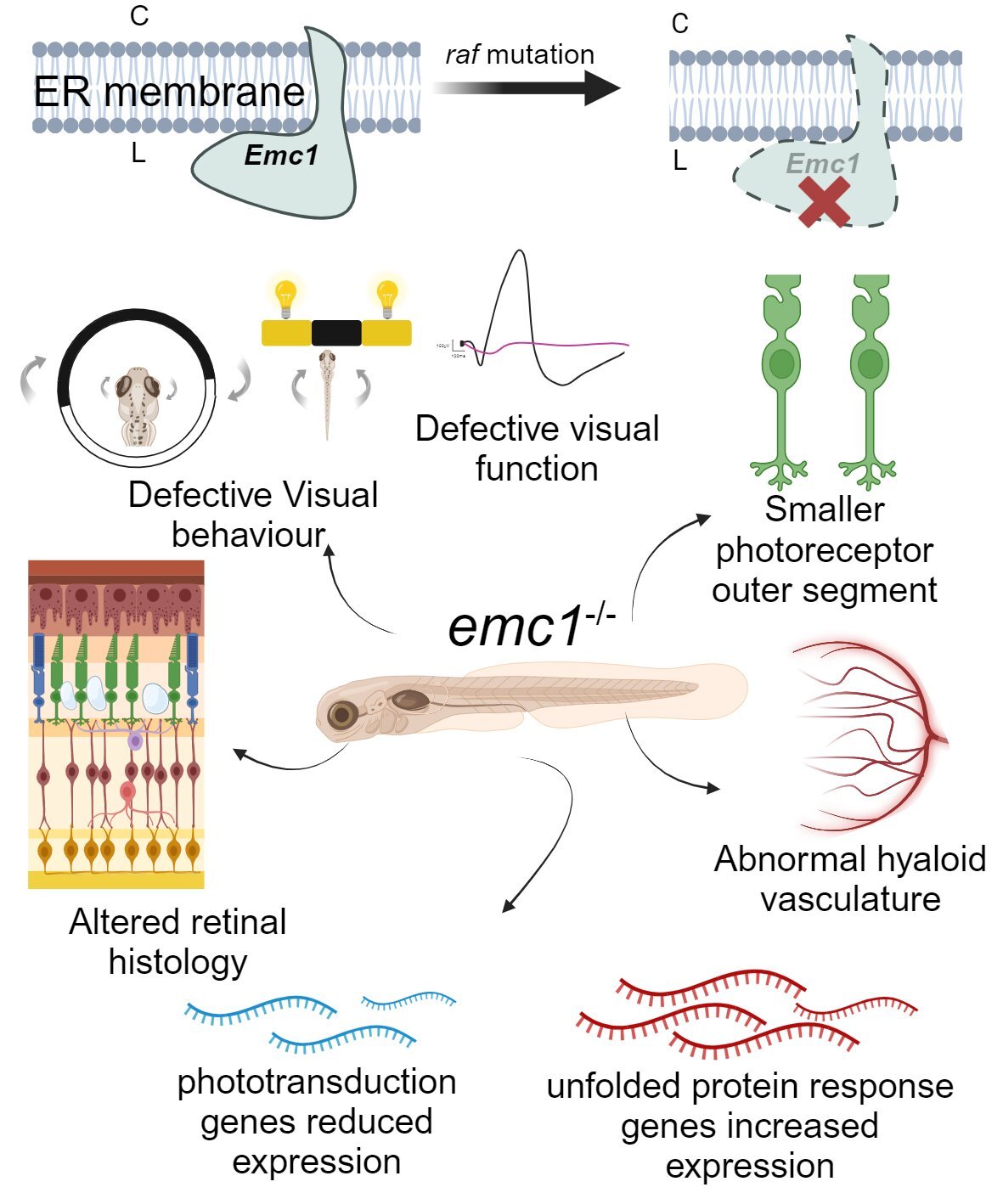
The research team, led by postdoctoral researcher Dr. Tess McCann, focused on the gene emc1, which plays an essential role in maintaining the proper functioning of the endoplasmic reticulum (ER) in cells. The ER is involved in the production and transport of proteins, which are vital for various cellular functions. Mutations in the emc1 gene are linked to eye diseases, particularly inherited retinal conditions that result in progressive vision loss.
Zebrafish, small tropical fish commonly used in biomedical research due to their biological similarities to humans, served as the model organism for this study. To identify genes that could be linked to blindness, the researchers created random mutations across the zebrafish genome. The offspring of these zebrafish were then screened for visual impairments, and one family stood out for exhibiting traits consistent with blindness. These zebrafish carried a mutation in the emc1 gene and were subsequently named the “raifteirí” (raf) zebrafish, after the 18th-century blind Irish poet Antoine Ó Raifteirí. The name symbolizes both the visual impairment caused by the mutation and the cultural heritage of Ireland, emphasizing hope for future advances in treating inherited blindness.
The findings were published in The FASEB Journal, shedding light on the role of the emc1 gene in retinal health. Zebrafish with the emc1 mutation displayed several critical issues in their vision. They had poorly developed eye cells that were thinner than usual, with unusual shapes, and they failed to react to visual stimuli. Additionally, the genes responsible for processing light signals in the retina were not functioning properly. Dr. McCann explained that the raf zebrafish demonstrated major vision problems, with their photoreceptors particularly sensitive to the loss of emc1. This marked the first comprehensive study to assess visual behavior in zebrafish without emc1 and revealed how essential this gene is for maintaining normal retinal function.
This discovery is significant because inherited retinal diseases, though rare, often lead to progressive vision loss. These conditions are caused by the degeneration of retinal cells, which impairs the ability to detect light and results in blindness. Over 300 genes have been identified in human patients with inherited retinal diseases, but the exact mechanisms behind these genetic mutations are not always well understood. The emc1 gene mutation in the zebrafish provides a valuable model for studying the causes of these diseases and could help identify potential solutions for restoring sight.
Currently, there is only one approved therapy for inherited retinal diseases, but it is only effective for a small subset of patients. Many other forms of inherited blindness still have no known cure or effective treatment. This gap in treatment options highlights the need for continued research into the genetic underpinnings of retinal diseases and the development of novel therapies.
Zebrafish have proven to be an invaluable tool in this area of research. Their biological makeup shares many similarities with humans, making them ideal subjects for studying genetic diseases. They also have transparent embryos, which allow scientists to observe developmental processes in real-time. In this study, the raf zebrafish model enabled the researchers to observe the effects of the emc1 mutation on vision and retinal health in a way that had not been possible before.
Professor Breandan Kennedy, who led the research team at UCD, emphasized the significance of this new zebrafish model. He noted that this is the first vertebrate animal model with a complete loss of emc1, providing a unique opportunity to study eye diseases and discover potential treatments. The researchers plan to continue exploring the effects of emc1 mutations, including those already linked to human patients but not yet fully understood. Additionally, they will test previously identified pharmacological compounds to see if they can restore vision in these zebrafish, offering hope for similar therapeutic approaches in humans.
The naming of the emc1-mutated zebrafish as “raifteirí” reflects the researchers’ respect for both scientific rigor and cultural heritage. In scientific naming, there is often an effort to convey something about the function or character of the gene or organism being studied. By naming the zebrafish after Antoine Ó Raifteirí, the blind Irish poet, the team acknowledges the human aspect of their research while also honoring Ireland’s cultural identity. This naming choice also symbolizes hope for the future, as the team believes that their findings could one day lead to treatments for inherited blindness, offering a brighter future for those affected by these conditions.
The research on emc1 in zebrafish not only advances our understanding of retinal diseases but also highlights the potential for using animal models to study human conditions. The connection between zebrafish and human biology continues to be a powerful tool for uncovering the genetic causes of diseases and finding new therapeutic strategies. This study serves as a reminder of the importance of model organisms in biomedical research and the potential they offer in advancing our knowledge and treatment options for human diseases.
Reference: Tess McCann et al, Emc1 is essential for vision and zebrafish photoreceptor outer segment morphogenesis, The FASEB Journal (2024). DOI: 10.1096/fj.202401977R