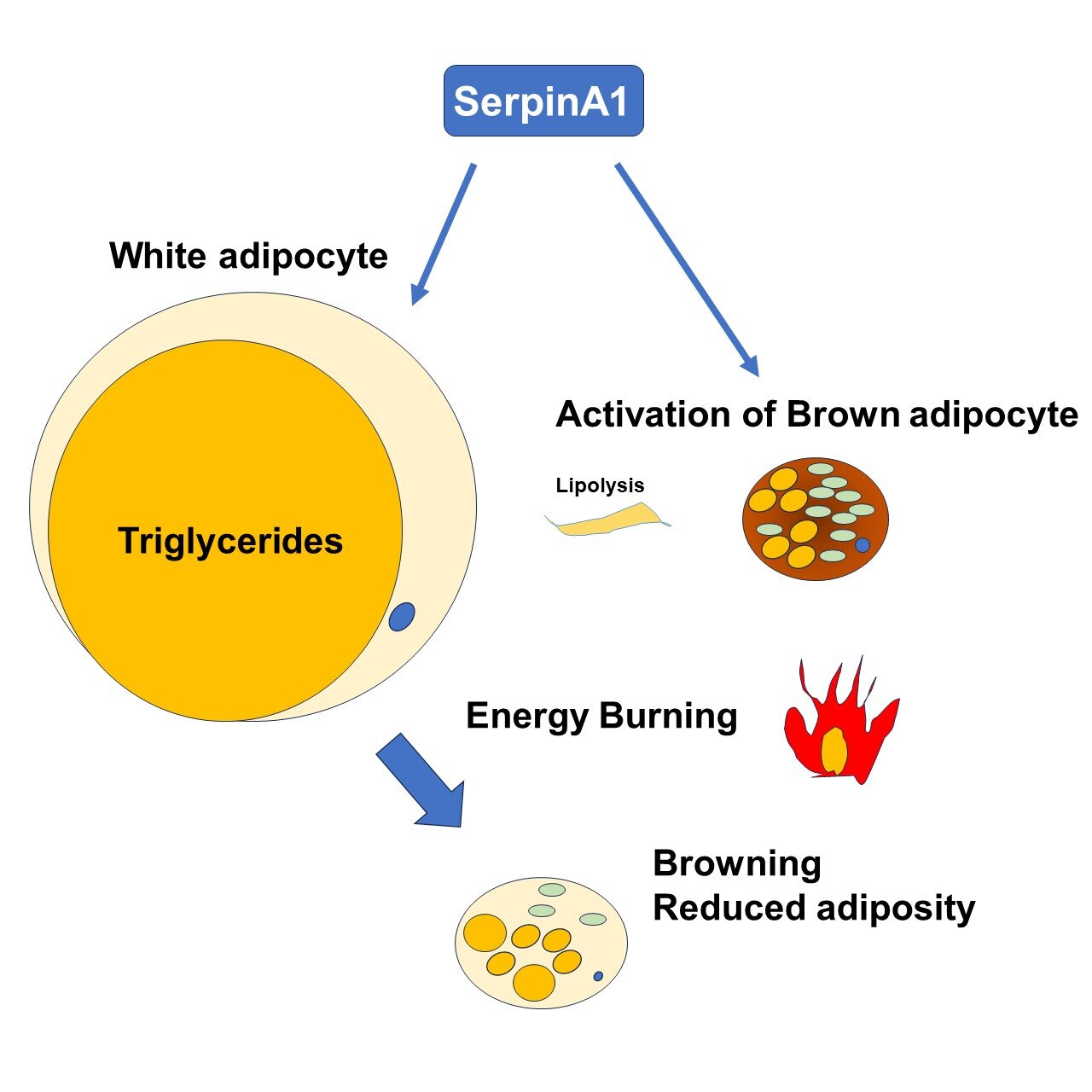
Researchers from Kumamoto University have made a groundbreaking discovery in the fight against obesity and metabolic disorders. Their study, published in Nature Communications, identifies a liver-derived protein called serine protease inhibitor A1 (SerpinA1) as a crucial regulator for enhancing energy expenditure and improving glucose metabolism. By focusing on the activation of brown adipose tissue (BAT) and the browning of white adipose tissue (WAT), the researchers have opened up promising avenues for innovative therapies targeting obesity and type 2 diabetes.
Adipose tissue, commonly referred to as fat, exists in two major forms in the human body: white adipose tissue (WAT) and brown adipose tissue (BAT). While WAT primarily functions as a storage depot for excess energy in the form of lipids, BAT plays the opposite role—it burns stored energy to generate heat, a process known as thermogenesis. The thermogenic activity of BAT is mediated by a key protein called uncoupling protein 1 (UCP1), which allows mitochondria to release energy as heat. This mechanism is especially significant because it provides a natural way to counteract weight gain and maintain energy balance.
One of the challenges associated with BAT lies in its decline as individuals age. This decline reduces the body’s ability to burn calories, thereby increasing the risk of obesity and metabolic disorders. Furthermore, obesity is often accompanied by the accumulation of WAT, which exacerbates health problems such as insulin resistance and type 2 diabetes. Researchers have long been searching for ways to stimulate BAT activity or convert WAT into BAT-like cells, a phenomenon termed “browning,” to address these metabolic challenges.
The study by Kumamoto University sheds new light on the role of SerpinA1 in this context. SerpinA1, a protein secreted by the liver, was found to promote the browning of WAT while also enhancing BAT activity. The researchers discovered that SerpinA1 increases the expression of UCP1 in adipocytes, boosting their thermogenic capacity. The implications of these findings are significant, as they suggest that SerpinA1 could serve as a therapeutic target for combating obesity and related diseases.
Led by Assistant Professor Masaji Sakaguchi, the research team conducted a series of experiments to evaluate the role of SerpinA1 in energy metabolism. They used transgenic mice engineered to overexpress SerpinA1 and observed remarkable results. These mice exhibited increased energy expenditure, better glucose tolerance, and resistance to weight gain, even when fed a high-fat diet. Notably, the mice displayed an upregulation of UCP1 and enhanced mitochondrial activity in their fat tissues, demonstrating the protein’s efficacy in promoting metabolic health.
Conversely, mice lacking SerpinA1 exhibited reduced mitochondrial activity, impaired thermogenesis, and increased susceptibility to obesity and insulin resistance. These findings highlight the crucial role of SerpinA1 in maintaining energy homeostasis and preventing metabolic disorders.
The mechanism by which SerpinA1 exerts its effects was also elucidated in the study. Researchers found that SerpinA1 interacts with a cell surface molecule called EphB2. This interaction activates signaling pathways that enhance UCP1 expression and mitochondrial activity in fat cells. Importantly, this pathway operates independently of the traditional β-adrenergic signaling mechanisms typically associated with thermogenesis. This independence makes SerpinA1 an especially attractive target for therapy, as it provides an alternative route for activating BAT and browning WAT without relying on existing pathways.
Assistant Professor Sakaguchi emphasized the potential therapeutic applications of these findings, stating, “Our research suggests that increasing SerpinA1 levels in the body could offer a novel approach to managing metabolic diseases like obesity and type 2 diabetes.” He highlighted that this strategy could harness the body’s natural fat-burning abilities, offering a sustainable and physiological solution to metabolic disorders.
The study’s implications extend beyond basic science, paving the way for clinical applications. With the prevalence of obesity and metabolic syndrome continuing to rise globally, innovative treatments are urgently needed. The discovery of SerpinA1’s role in regulating fat metabolism offers a new hope for individuals struggling with weight management and diabetes. The researchers are now focused on developing therapies that leverage this mechanism, with the goal of translating their findings into real-world interventions.
The potential clinical applications are vast. For instance, therapies targeting SerpinA1 could be developed as injectable drugs or small molecules that increase the protein’s activity in the liver. Such treatments could be particularly beneficial for patients who are resistant to existing weight-loss drugs or those who experience adverse effects from traditional therapies. Moreover, because the SerpinA1 pathway operates independently of the β-adrenergic system, it could be used in conjunction with other metabolic treatments for a synergistic effect.
This discovery also holds promise for improving glucose metabolism in patients with diabetes. By enhancing mitochondrial activity and promoting the browning of WAT, SerpinA1 could help normalize blood sugar levels and improve insulin sensitivity. These effects would be particularly advantageous for individuals with type 2 diabetes, a condition closely linked to obesity and metabolic dysfunction.
Looking ahead, the research team is planning further studies to explore the safety and efficacy of SerpinA1-based therapies in humans. They are also investigating whether variations in SerpinA1 levels or activity could explain individual differences in metabolic health, providing a foundation for personalized treatment approaches.
The study underscores the importance of understanding the intricate relationships between the liver, adipose tissue, and overall metabolism. It highlights how the liver, traditionally viewed as an organ primarily involved in digestion and detoxification, also plays a central role in regulating energy balance and metabolic health.
Reference: Shota Okagawa et al, Hepatic SerpinA1 improves energy and glucose metabolism through regulation of preadipocyte proliferation and UCP1 expression, Nature Communications (2024). DOI: 10.1038/s41467-024-53835-9
Think this is important? Spread the knowledge! Share now.