UCSF scientists have made a groundbreaking discovery by identifying a stem cell in the developing human brain that is capable of forming cells found in deadly brain tumors, such as glioblastoma. This finding is not only pivotal for understanding brain cancer but also opens a window into the mechanics of human brain development and disorders like autism. The research, which represents one of the most comprehensive genomic surveys of human brain cells during the first two decades of life, has been published in the prestigious journal Nature.
The foundation of the study lies in understanding how the human brain grows and matures, particularly focusing on developmental stages that may hold keys to understanding neurological diseases. Arnold Kriegstein, MD, Ph.D., one of the lead authors, emphasized that many brain disorders have their origins in development. Yet, until now, there has not been a detailed roadmap outlining normal brain development. The new research provides this crucial map, highlighting the genetic programs that are active in different stages of growth and showing how these can sometimes go awry to contribute to diseases.
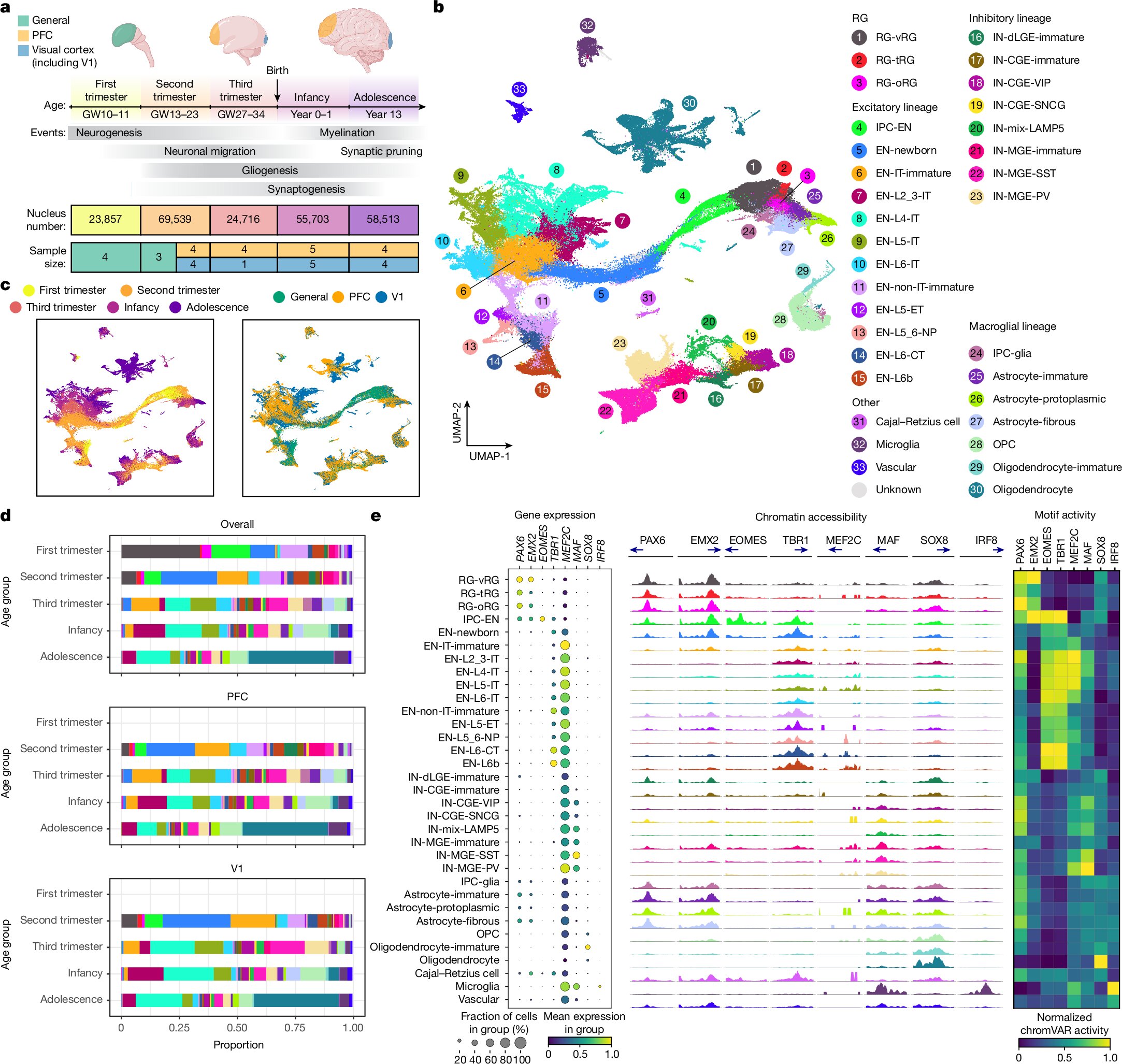
The scientists meticulously analyzed cells taken from donated brain tissues, tracking their original location in the brain. This allowed them to study the role of gene expression in creating the brain’s intricate architecture and connections. By examining the RNA in these cells—molecules that serve as the intermediary between DNA and the proteins that perform most biological functions—the researchers gained a deep insight into how the brain’s developmental programs operate.
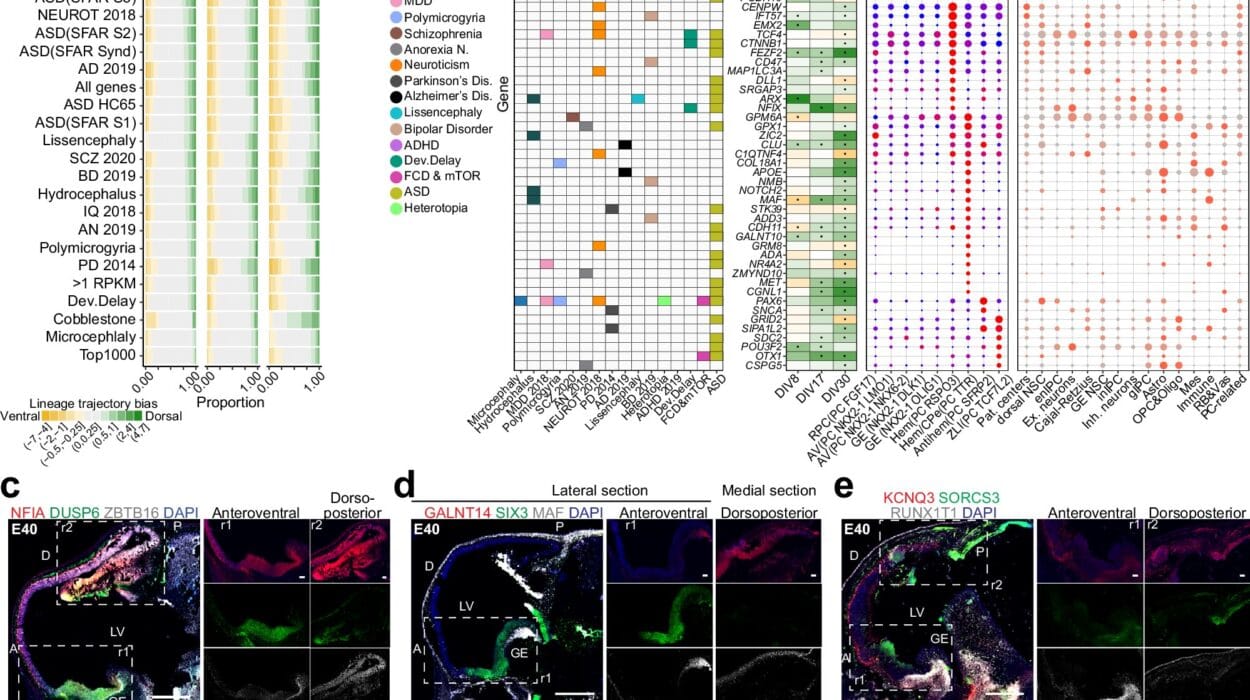
One of the key findings of this study is the identification of a unique stem cell capable of developing into three distinct cell types found in glioblastoma. Most stem cells in the developing brain have a limited lineage, maturing into just one or two types of cells, such as neurons or support cells like glia. However, this particular stem cell defied the norm, showing the potential to differentiate into two types of glial cells and one type of neuron. This unexpected discovery lends strong support to the theory that brain cancers, including glioblastoma, hijack these developmental programs to enable their rapid and heterogeneous growth later in life.
The findings suggest that glioblastoma might originate from this multipotent stem cell, which retains its ability to recreate the diversity of cells in the tumor. Understanding how this stem cell behaves during development could pave the way for new treatments targeting the tumor’s origin. If scientists can disrupt the process through which this stem cell fosters glioblastoma growth, they could potentially develop more effective therapies.
Equally intriguing are the study’s implications for autism, a disorder that arises from the interaction of multiple genetic factors rather than a single mutation. The researchers found that many genes associated with autism are active in immature neurons during early development. These neurons, which are still migrating through the developing brain and forming connections, rely on complex genetic programs to determine their proper placement and function. Mutations affecting these genes might interfere with these early developmental processes, potentially contributing to the emergence of autism.
Although the study did not directly examine tissue from individuals with autism, the findings provide valuable clues about the disorder’s origins. By linking specific genetic variations to the cells that serve as building blocks of the young brain, the research helps to map the “dots” that underlie autism’s development. Kriegstein noted that further investigation during this critical developmental phase could yield profound insights into the disorder and guide the creation of novel interventions.
The study’s innovative methodology also deserves recognition. Unlike traditional research that often relies on animal models as proxies for human development, this work directly analyzed human brain tissues. Researchers collaborated with the NIH NeuroBioBank and UCSF-associated hospitals to obtain high-quality brain samples donated from 27 individuals spanning early life to adolescence. By employing cutting-edge genomic techniques, the team analyzed gene expression and chromatin accessibility—the open areas of DNA where genes can be activated. The result is a high-resolution map of brain development that is not only comprehensive but also unique to humans, as the animal models previously used fail to replicate critical aspects of human biology.
Li Wang, Ph.D., co-first and co-corresponding author of the study, noted the importance of measuring both RNA and chromatin states within individual cells. By mapping these molecular markers back to specific brain structures, the researchers could construct an intricate narrative of how the brain develops over time. The samples, drawn from key regions like the front and back of the cerebral cortex, yielded data that reveals how learning, memory, and language circuits begin to form.
One of the significant technical challenges the researchers overcame was the instability of RNA. Since RNA molecules degrade quickly, only pristine tissue samples can yield meaningful data. The success of the research hinged on both the quality of the samples and the meticulous genomic analysis performed by the team. The broader neuroscience community also plays a vital role in this endeavor, as these brain donations represent a valuable and irreplaceable resource for advancing our understanding of brain development and disorders.
The discovery of the unique brain stem cell offers immense potential for developing new cancer treatments. Researchers theorize that by understanding the early context in which this stem cell gives rise to multiple cell types, they may be able to prevent its later co-option by tumor growth mechanisms. Glioblastoma, being one of the most aggressive and treatment-resistant brain cancers, could benefit significantly from targeted approaches that disrupt these developmental pathways.
Moreover, the findings related to autism suggest that addressing gene expression issues in developing neurons could become an important therapeutic strategy. By identifying when and how specific autism-associated genes become active during brain growth, scientists can better target the critical windows during which interventions might have the most impact.
The UCSF team has made their extensive data publicly available, recognizing its potential to answer a myriad of other scientific questions. Their work establishes a foundation for studying not just brain cancer and autism, but also other neurological and developmental disorders that emerge from the complex interplay of genetics and brain development.
This pioneering research underscores the intricate connections between early brain development and lifelong neurological health. By uncovering how the same genetic programs that drive healthy growth can be co-opted into pathological processes, the UCSF study opens new avenues for understanding and treating some of the most challenging brain disorders. The results demonstrate the power of combining cutting-edge genomics with direct human tissue analysis, setting a new standard for how neuroscience can address pressing medical questions. Scientists across the globe are now better equipped to explore the mysteries of the human brain, thanks to this detailed and transformative roadmap.
Reference: Li Wang et al, Molecular and cellular dynamics of the developing human neocortex, Nature (2025). DOI: 10.1038/s41586-024-08351-7