For decades, scientists have theorized about how complex organic molecules form in the vast, cold emptiness of interstellar space. Among these molecules, benzene has been a subject of particular interest due to its role as a potential precursor to polycyclic aromatic hydrocarbons (PAHs). These PAHs are believed to be crucial carriers of cosmic carbon, which may have played a fundamental role in the origins of carbon-based life. However, a recent experiment conducted by astrophysicists at the University of Colorado’s JILA, in collaboration with the National Institute of Science and Technology (NIST), has upended previous assumptions about how benzene is formed in space.
The study, published in Nature Astronomy, was led by G. S. Kocheril, C. Zagorec-Marks, and H. J. Lewandowski. Their findings indicate that a long-standing theoretical model of benzene formation may be incorrect, forcing scientists to reevaluate how these vital molecules emerge in interstellar environments.
The Importance of Benzene in Cosmic Chemistry
Benzene is one of the simplest aromatic hydrocarbons, consisting of a ring of six carbon atoms bonded to hydrogen atoms. Its presence in space is of great interest because it is thought to be a fundamental building block in the formation of larger, more complex organic molecules. PAHs, which arise from benzene-like structures, have been detected in meteorites and interstellar dust clouds. These molecules are hypothesized to be essential in the chemistry that led to the emergence of life on Earth.
Previous research in the 1990s proposed that benzene could form in space through ion-molecule reactions, where positively charged ions interact with neutral molecules in the extreme conditions of the interstellar medium. This theory suggested that acetylene (C₂H₂) could react with a proton donor, such as N₂H⁺ (diazenylium), to produce benzene in cold, low-pressure environments. However, this hypothesis had never been directly tested in conditions simulating interstellar space—until now.
The Experiment That Defied Expectations
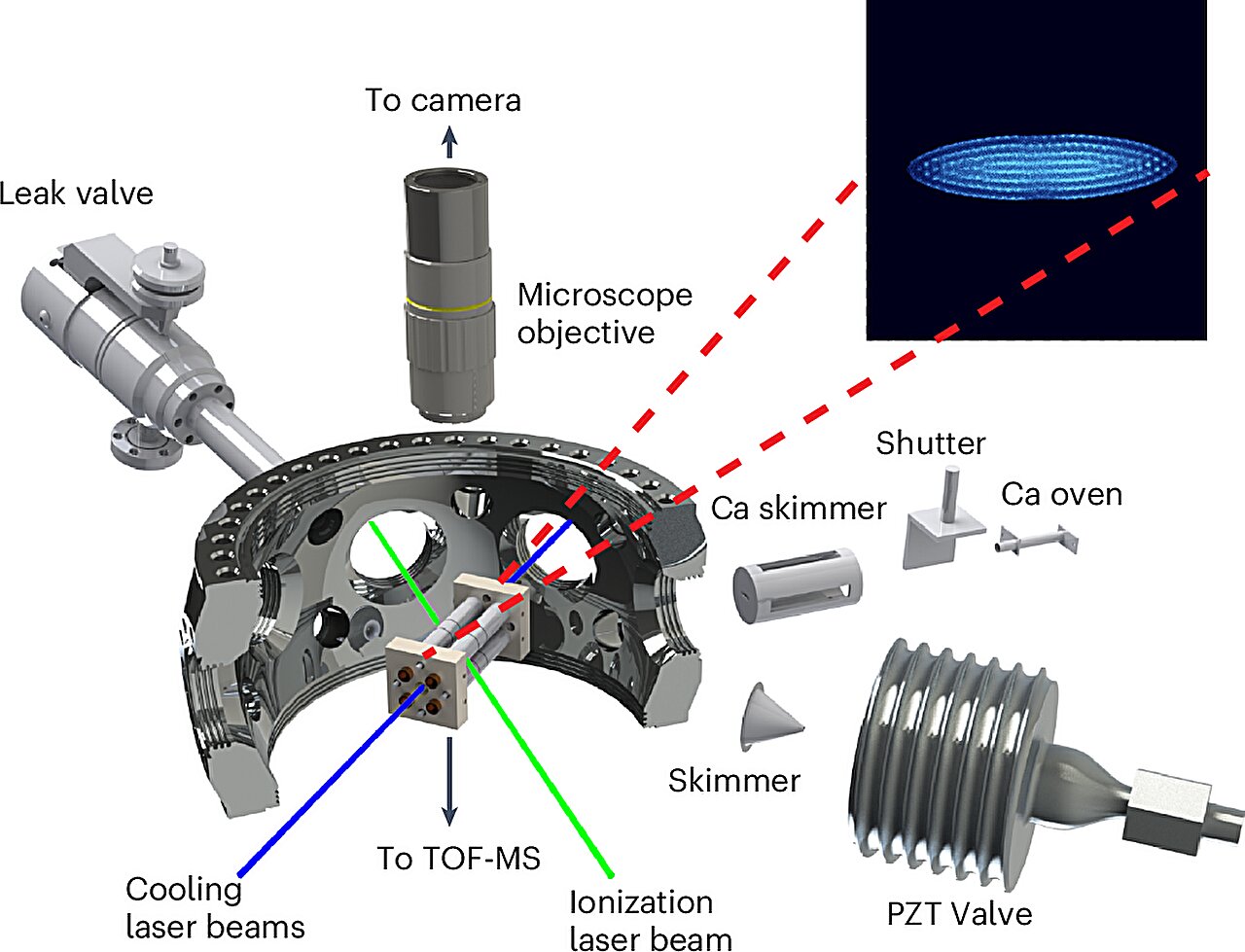
Creating the conditions of interstellar space in a laboratory is an immense challenge. The vast emptiness of space is characterized by near-zero temperatures and ultra-low pressures that are difficult to replicate on Earth. However, the JILA research team possessed the necessary equipment to attempt this feat.
Their experiment involved cooling their test environment to just 1 Kelvin (-272°C), almost reaching absolute zero, and reducing the pressure to a trillionth of Earth’s sea-level atmospheric pressure. These extreme conditions mimicked those found in interstellar clouds, where chemical reactions occur at an incredibly slow rate.
The researchers introduced neutral acetylene molecules and exposed them to the proton donor N₂H⁺, monitoring the reactions using a highly sensitive mass spectrometer. As expected, the acetylene molecules were protonated, forming C₆H₅⁺ ions. According to the prevailing theory, adding molecular hydrogen (H₂) at this stage should have resulted in the formation of benzene.
Surprisingly, that reaction did not occur. The expected chemical pathway for benzene formation failed to materialize, meaning that no aromatic ring was created. This result challenges the idea that ion-molecule reactions drive benzene synthesis in interstellar environments.
Implications for Our Understanding of Interstellar Chemistry
The findings from this experiment have profound implications for astrochemistry. If benzene is not formed through the previously assumed ion-molecule reaction pathway, scientists must reconsider how PAHs and other complex carbon molecules emerge in space. This could reshape our understanding of the chemical processes that contribute to the formation of organic compounds essential for life.
One possible alternative explanation lies in earlier research conducted at the University of Hawaii in 2011. That study suggested that neutral-neutral reactions—specifically, interactions between ethynyl radicals (C₂H) and 1,3-butadiene (C₄H₆)—could lead to benzene formation. Unlike ion-molecule reactions, neutral-neutral reactions do not rely on charged particles, potentially offering a more viable pathway for benzene synthesis in the cold, diffuse regions of space.
Additionally, the failure of the ion-molecule reaction suggests that scientists need to explore other mechanisms, such as photochemical reactions driven by ultraviolet radiation or interactions involving cosmic rays. These alternative pathways could provide insights into the origins of aromatic hydrocarbons and their distribution throughout the universe.
A New Direction for Astrochemical Research
This unexpected result opens new avenues for research into the chemistry of interstellar molecules. The JILA team’s findings demonstrate that even long-standing theories can be overturned when subjected to rigorous experimental testing. Moving forward, scientists will need to refine their models of molecular formation in space, incorporating alternative reaction pathways that align with observational data.
Future experiments may involve testing other potential formation routes for benzene and PAHs under interstellar-like conditions. Advanced telescopic observations of molecular clouds, combined with laboratory studies, will be crucial in identifying the true chemical pathways at work in space.
The quest to understand cosmic chemistry is far from over. While this experiment has ruled out one prominent theory, it has also paved the way for new discoveries that may ultimately reveal how the fundamental building blocks of life emerged in the universe.
Reference: G. S. Kocheril et al, Termination of bottom-up interstellar aromatic ring formation at C6H5+, Nature Astronomy (2025). DOI: 10.1038/s41550-025-02504-y