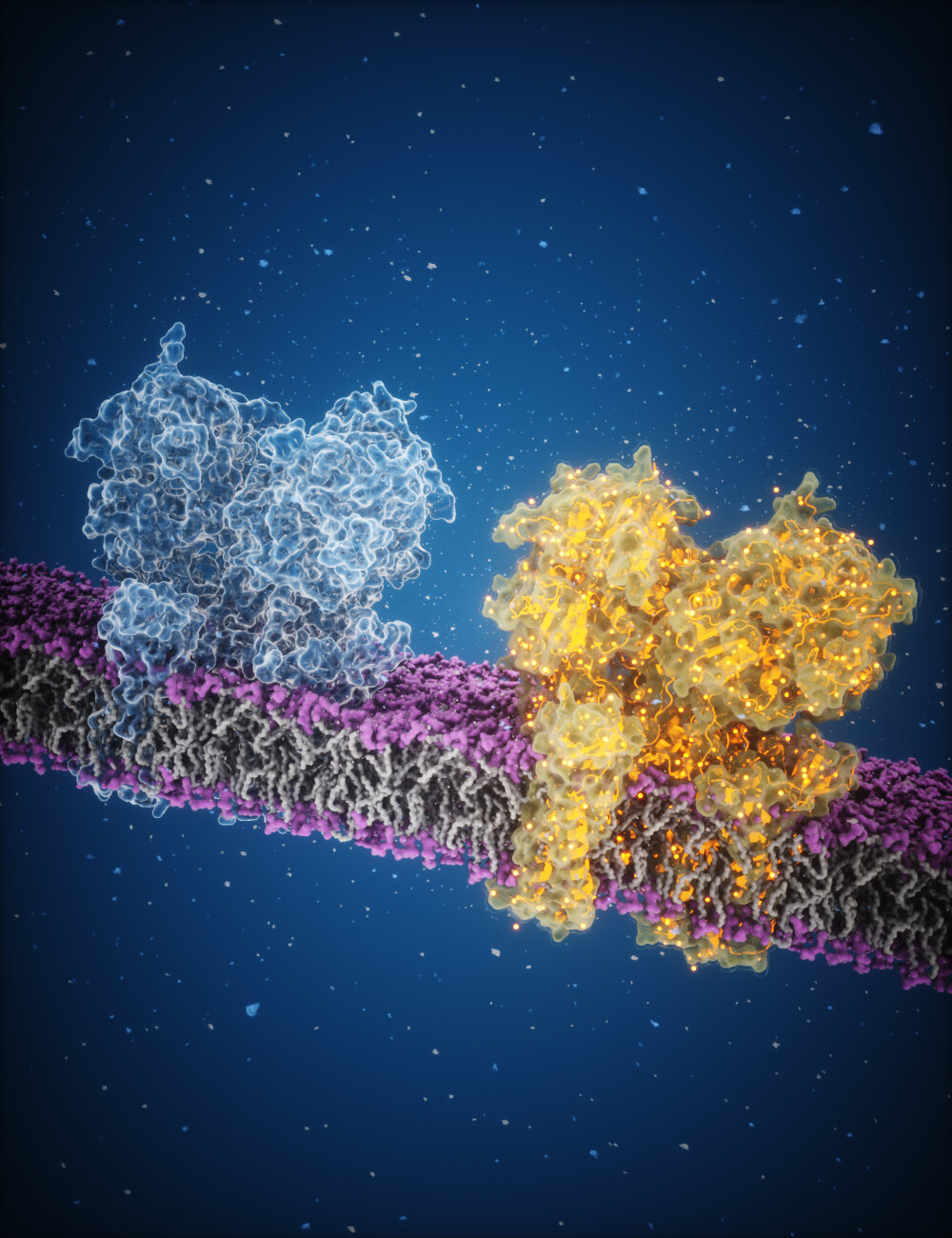
The human brain is an intricate network of neurons, constantly exchanging chemical messages that govern thought, memory, movement, and emotion. At the core of this communication lies glutamate, one of the most common and powerful neurotransmitters. Despite its importance, scientists have struggled to capture the precise molecular details of how glutamate interacts with its receptor to trigger neuronal activity.
In a groundbreaking study led by researchers at Johns Hopkins Medicine, scientists have now visualized this process in unprecedented detail. Using advanced cryo-electron microscopy (cryo-EM), they have uncovered how glutamate binds to AMPA receptors—channels that allow charged particles to enter neurons and generate electrical signals. Their findings, published in Nature, provide valuable insights that could pave the way for new treatments for conditions such as epilepsy and intellectual disorders.
The Role of Glutamate in Neural Signaling
Glutamate plays a fundamental role in brain function, acting as a key messenger in neuronal communication. When released into the synapse—the gap between neurons—glutamate binds to specialized proteins on the receiving neuron, triggering a cascade of electrical signals. One of its primary targets is the AMPA receptor, a channel that operates like a gate, allowing charged particles to flow into the cell. This movement generates electrical impulses, which are essential for learning, memory, and sensory perception.
Dysfunction in AMPA receptors has been linked to various neurological disorders. In conditions like epilepsy, excessive activation of these receptors can lead to uncontrolled electrical activity, resulting in seizures. Understanding how glutamate interacts with AMPA receptors at the molecular level could lead to new therapies that regulate these signals more precisely.
Capturing the Molecular Dance with Cryo-EM
To study how AMPA receptors function, researchers turned to cryo-electron microscopy, a revolutionary imaging technique that enables scientists to observe molecular structures at near-atomic resolution. Traditionally, studying receptor activity has been challenging because these proteins are highly dynamic and change shape rapidly. By freezing them in place at different moments, cryo-EM allows researchers to capture snapshots of their movements.
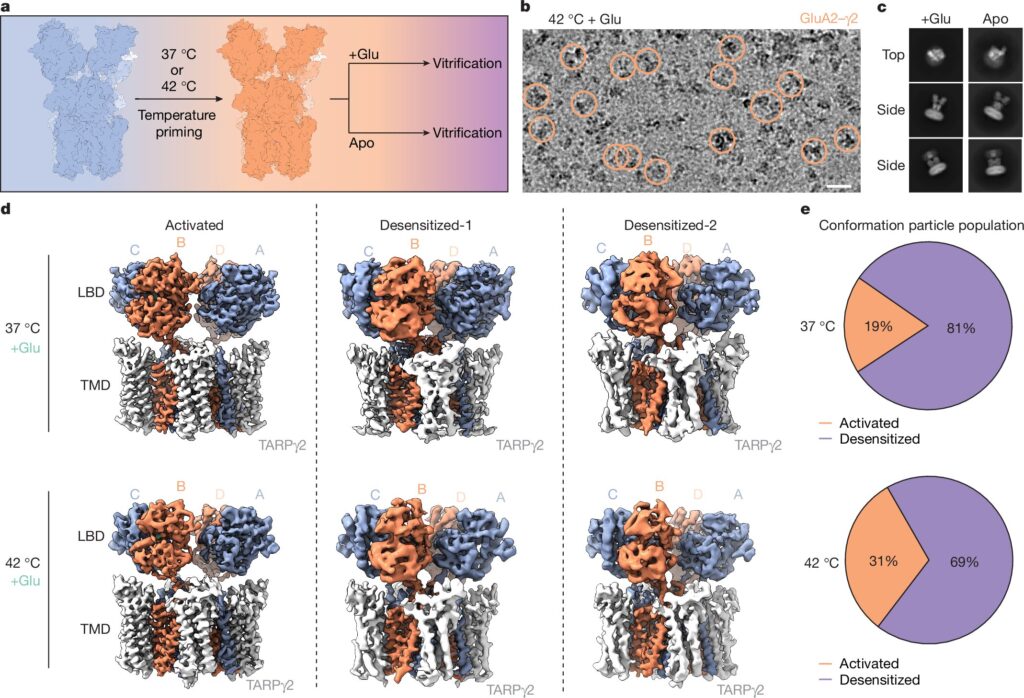
For this study, the researchers began by purifying AMPA receptors from human embryonic cells grown in the lab. These receptors were then heated to body temperature (37°C or 98.6°F), mimicking their natural environment in the brain. Next, they exposed the receptors to glutamate and quickly flash-froze them to preserve the interactions in their native state.
By assembling over a million images taken with cryo-EM, the team reconstructed a detailed 3D map of how AMPA receptors respond to glutamate binding. The images revealed a critical mechanism: when glutamate binds, the AMPA receptor undergoes a dramatic structural change, closing around the molecule like a clamshell. This action pulls open the channel, allowing ions to rush into the neuron and generate an electrical signal.
Implications for Neurological Disorders and Drug Development
These new insights have significant implications for the development of drugs targeting AMPA receptors. Current epilepsy medications, such as perampanel, work by blocking AMPA receptors to reduce excessive neuronal activity. The study confirms that these drugs act like “door stoppers,” preventing the channel from opening fully.
However, the ability to precisely control AMPA receptor activity opens new possibilities for drug design. Instead of simply blocking the receptor, future drugs could fine-tune its activity—either enhancing or reducing its function depending on the disorder being treated. For example, in neurodevelopmental disorders where AMPA receptor function is impaired, new compounds could be designed to boost their activity and improve synaptic signaling.
By mapping the step-by-step process of receptor activation, researchers can now explore ways to design more selective and effective treatments. This approach could lead to new therapies for a range of neurological conditions, including Alzheimer’s disease, schizophrenia, and stroke recovery.
Advancing Our Understanding of Brain Function
Beyond its medical applications, this research represents a significant milestone in neuroscience. Each discovery brings scientists closer to unraveling the complex biochemical interactions that enable thought, learning, and perception. By visualizing how neurotransmitters like glutamate control brain activity at the atomic level, researchers are laying the foundation for future breakthroughs in understanding consciousness and cognition.
According to lead researcher Edward Twomey, Ph.D., these findings are part of a broader effort to decode the fundamental mechanisms of brain function. “With each new finding, we are figuring out each of the building blocks that enable our brains to function,” he explains.
The study also highlights the power of cutting-edge imaging technologies like cryo-EM. As this technique continues to evolve, it promises to reveal even deeper insights into the molecular machinery of the brain, potentially unlocking new ways to treat a host of neurological conditions.
A New Frontier in Brain Science
The ability to observe molecular events in such detail marks an exciting era for neuroscience. The discovery of how glutamate opens AMPA receptors provides a crucial missing piece in the puzzle of neural communication. As researchers continue to explore this intricate system, their findings could lead to revolutionary treatments for neurological and psychiatric disorders, offering hope to millions affected by conditions linked to disrupted synaptic signaling.
By peering into the hidden world of brain chemistry, scientists are not only advancing medicine but also deepening our understanding of what makes us think, feel, and experience the world around us.
Reference: Anish Kumar Mondal et al, Glutamate gating of AMPA-subtype iGluRs at physiological temperatures, Nature (2025). DOI: 10.1038/s41586-025-08770-0