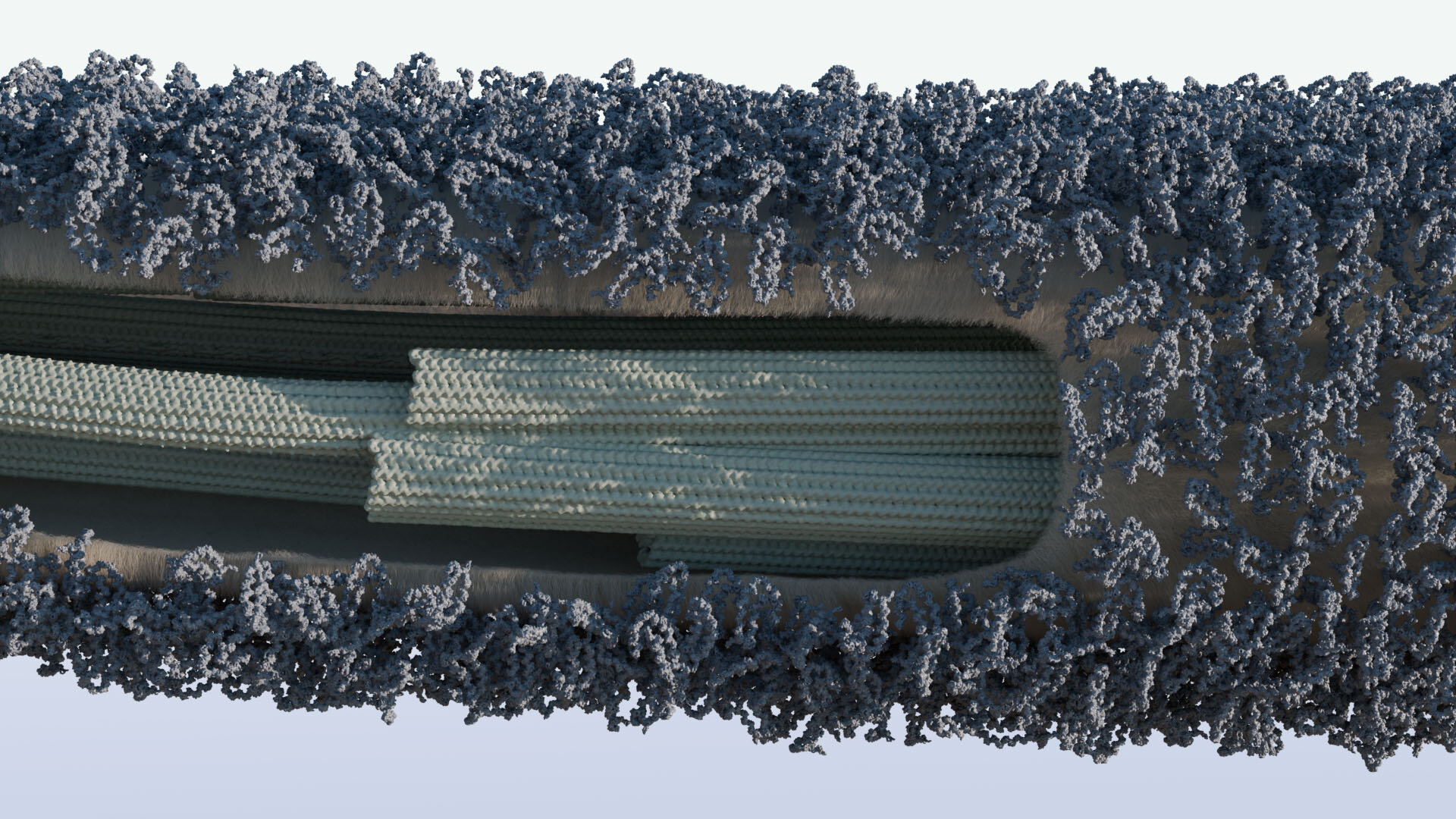
If you were to zoom in on a single living cell, you might be surprised by its complexity. Among its most intriguing features are tiny, hair-like structures called cilia, protruding from the cell’s surface. These delicate projections, often swaying in unison, perform essential roles: they move cells through fluid, sense the surrounding environment, and even help cells stick to surfaces. But what protects these fragile cellular appendages as they brave the microscopic world? Scientists have long suspected there was an invisible shield, but only now are we beginning to understand its true nature.
In an exciting breakthrough, an international team of researchers from Germany and Italy has uncovered the fine details of this protective covering—the glycocalyx—that envelops cilia in a model organism called Chlamydomonas reinhardtii, a unicellular green alga. Their study, recently published in Advanced Science, reveals the specific molecules responsible for safeguarding these vital structures and sheds light on ancient, shared biological strategies that stretch across the tree of life.
A Sugar-Coated Shield with Big Responsibilities
Cilia are more than just passive feelers or microscopic paddles; they are dynamic sensory and mechanical machines. Whether they’re helping single-celled organisms navigate their aquatic worlds or clearing mucus from human lungs, cilia are on the front lines of cellular interaction with the environment. This makes them vulnerable to everything from mechanical stress to potentially harmful chemical signals.
That’s where the glycocalyx comes in. Literally meaning “sugar coat,” this sheath is a complex network of sugar-rich molecules called glycoproteins. While it was known that this sugary armor existed on cilia, its precise composition and function had remained something of a mystery—until now.
The Discovery: Two Glycoproteins Take Center Stage
Using a suite of sophisticated imaging and biochemical techniques, the team focused on Chlamydomonas reinhardtii, a single-celled alga frequently used as a model for studying cilia because of its simplicity and well-mapped genome. What they found was striking: two glycoproteins, dubbed FMG1B and a newly identified counterpart, FMG1A, form the core structure of the algal ciliary glycocalyx.
These glycoproteins are more than just structural components. Their architecture shares a surprising resemblance to mucins, the gooey proteins that make up the mucus coating many animal tissues, from our lungs to our digestive tracts. Mucins are known for their protective functions—forming a slippery barrier against pathogens, toxins, and mechanical damage—so finding mucin-like glycoproteins on algal cilia was an unexpected connection. It suggests that the strategy of using sugary, protective proteins to interface with the environment is both ancient and widely conserved across life forms.
Sticky Situation: What Happens Without the Shield?
To understand exactly what FMG1A and FMG1B do, the scientists employed genetic manipulation to remove them from the alga’s genome. What they observed was remarkable. Without these glycoproteins, the cilia became significantly stickier. One might imagine this stickiness would make it easier for the algae to cling to surfaces, but the opposite was true.
Despite the increased adhesiveness, the algae still managed to crawl and glide along surfaces—abilities they were already known for. This result challenged the longstanding assumption that FMG1A and FMG1B were directly involved in adhesion and motility. Instead, the findings point to a more nuanced role: these glycoproteins form a protective layer that fine-tunes how sticky the cilia are, preventing them from adhering too strongly and potentially becoming immobilized.
“This discovery expands our knowledge of how cells regulate direct interaction with their environment,” explained Professor Michael Hippler, a plant biotechnologist from the University of Münster and co-author of the study. “We are also gaining insights into how similar protective mechanisms might work in other organisms,” added Dr. Adrian Nievergelt from the Max Planck Institute of Molecular Plant Physiology in Potsdam.
Peering into the Microscopic World with Advanced Technology
The research relied on a combination of cutting-edge technologies, including cryogenic electron tomography and electron microscopy, to visualize the ciliary surface at near-atomic resolution. These methods allowed the team to see the glycocalyx in its natural state, something that traditional microscopy could never have achieved.
In parallel, fluorescence microscopy helped track the presence and distribution of FMG1A and FMG1B across the algal surface, while mass spectrometry identified the chemical composition of the glycoproteins. Genetic engineering techniques enabled the researchers to selectively delete the genes encoding these proteins, revealing their crucial roles.
Altogether, this multidisciplinary approach provided an unprecedented glimpse into the molecular machinery that protects and regulates cilia—a critical advance for cell biology.
Why It Matters: More Than Just Algae
At first glance, Chlamydomonas reinhardtii might seem like a curious choice for such a high-tech investigation. After all, it’s a microscopic green alga. But its simplicity makes it an ideal model for understanding basic biological principles that apply broadly, including in humans.
Cilia are found across the eukaryotic world, from single-celled organisms to complex animals. In humans, defects in cilia can lead to ciliopathies, a group of genetic disorders that affect kidney function, fertility, respiratory health, and more. Understanding how cilia interact with their environments—and how they avoid sticking too much or becoming damaged—could inform medical research aimed at treating these conditions.
Furthermore, the discovery of mucin-like proteins in algae hints at the evolutionary roots of mucus and other protective coatings. It’s a reminder that even the simplest life forms can offer profound insights into biology’s grand narrative.
Looking Ahead: More Mysteries to Uncover
While this study has answered important questions about the ciliary glycocalyx, it also raises new ones. What other molecules contribute to this protective layer? How do cilia adjust their stickiness in response to changing environmental conditions? Could similar protective glycocalyces exist on other cellular structures, like flagella or microvilli?
The researchers are optimistic. Collaborations between institutions such as the University of Münster, the Max Planck Institute, and the Human Technopole in Milan promise to push the boundaries of our knowledge even further.
As Dr. Gaia Pigino, leader of the collaborating research group at Human Technopole, puts it: “We are just beginning to unravel the complexities of how cells interface with their environment. There is still so much more to learn.”
Conclusion: The Silent Guardians of Cellular Life
This new understanding of the glycocalyx covering cilia in Chlamydomonas is more than just a scientific curiosity. It’s a testament to the intricate designs evolved by life to survive, thrive, and adapt. From the sticky mucins lining our lungs to the glycoprotein shields on microscopic algae, nature has refined protective barriers that are as elegant as they are essential.
For now, these silent guardians—FMG1A and FMG1B—continue to do their work, protecting cilia as they glide, sense, and explore the microcosm. And thanks to the perseverance of scientists, we are one step closer to understanding—and maybe even harnessing—their remarkable secrets.
Reference: Lara M. Hoepfner et al, Unwrapping the Ciliary Coat: High‐Resolution Structure and Function of the Ciliary Glycocalyx, Advanced Science (2025). DOI: 10.1002/advs.202413355
Think this is important? Spread the knowledge! Share now.