Piper longum, commonly known as long pepper, has long been revered in traditional medicine systems around the world, particularly in Chinese medicine, for its healing properties. Mature fruits of this climbing vine, native to the Indian subcontinent and Southeast Asia, are commonly used as a spice and in herbal formulations. The pepper’s therapeutic value spans from alleviating cold-related ailments to acting as a potent pain reliever. But beyond its well-documented traditional applications, the fruits of P. longum also harbor untapped potential, particularly for modern pharmaceutical use, thanks to the wealth of bioactive compounds they contain.
Despite a long history of use in folk medicine, the complex chemical composition of P. longum has posed a challenge for scientists in the systematic identification of its active ingredients. As with many traditional Chinese medicines, the intricacy of the plant’s bioactive compounds requires advanced techniques to efficiently isolate and identify novel therapeutic agents. However, recent scientific breakthroughs are beginning to reveal the true potential of P. longum’s therapeutic constituents, promising new avenues for medicinal research.
A Breakthrough in Alkaloid Discovery: The Role of Molecular Network-Based Dereplication
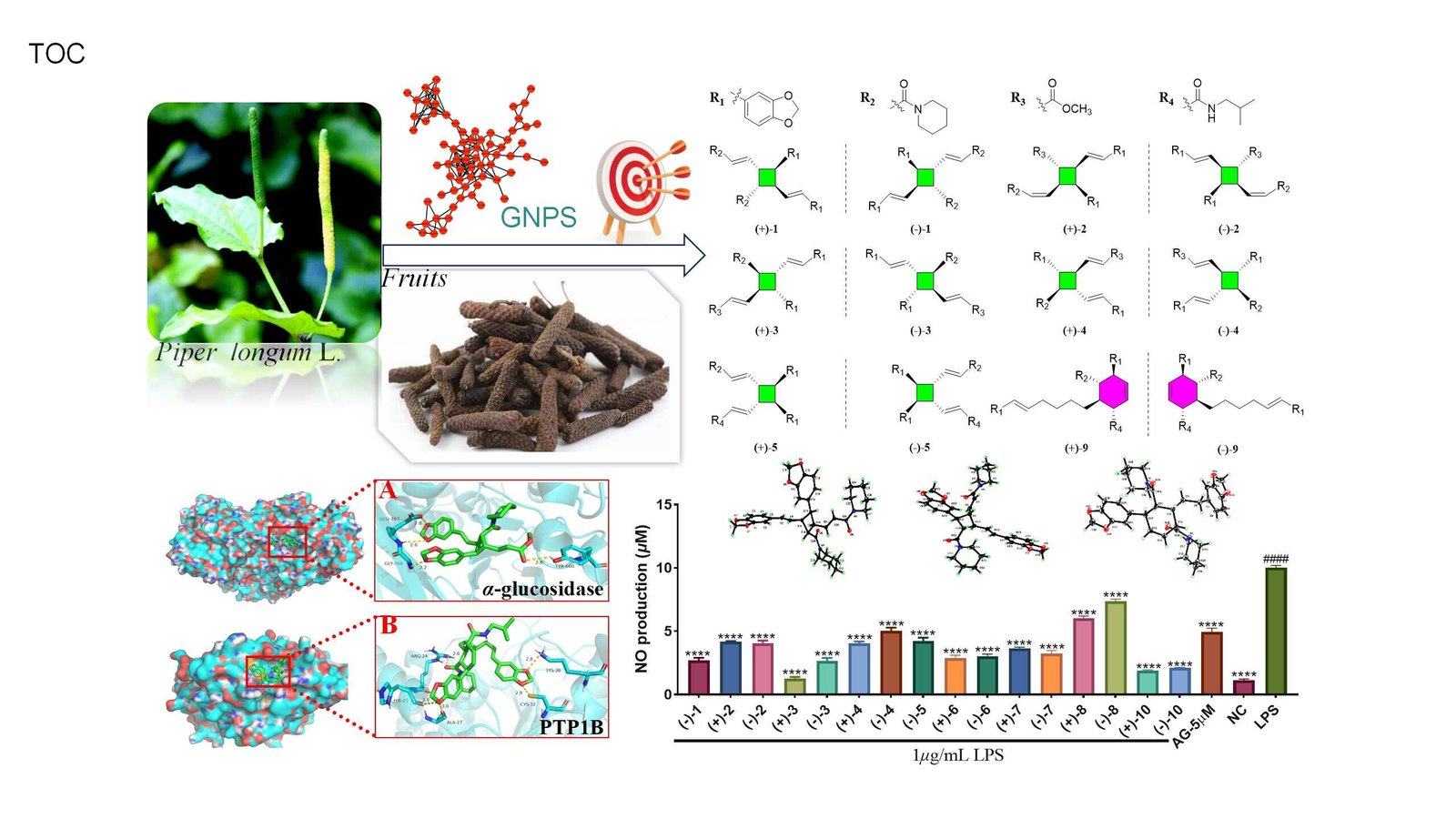
In a groundbreaking study, researchers led by Prof. Haji Akber Aisa from the Xinjiang Technical Institute of Physics & Chemistry of the Chinese Academy of Sciences have made a significant leap in unraveling the chemical mysteries of P. longum fruits. By employing an innovative molecular network-based dereplication strategy, the team successfully isolated 12 dimeric amide alkaloid enantiomers from the fruits, revealing their anti-inflammatory and antidiabetic effects. This research, which was published in the Journal of Agricultural and Food Chemistry, opens up exciting new possibilities for the therapeutic applications of P. longum.
Dereplication, the process of identifying known compounds in a sample, has become a powerful tool in natural product research. Given the complex chemical composition of traditional medicines, the ability to identify previously characterized molecules efficiently is essential for discovering new bioactive compounds. In this study, the team used the Global Natural Products Social Molecular Networking (GNPS) platform, a state-of-the-art tool that enables the creation of molecular networks based on the mass spectrometry (MS) profiles of plant extracts. This approach allowed them to quickly identify previously unknown compounds from the P. longum extract, laying the groundwork for their subsequent isolation and analysis.
Isolating Dimeric Amide Alkaloids: A New Class of Bioactive Compounds
The research team’s innovative approach led to the identification of a distinct molecular cluster within the network of a 95% ethanol extract of P. longum fruits. The cluster contained nodes with molecular weights ranging from 486.191 to 653.255 Da, which did not correspond to any known chemical structures in public databases, suggesting they were novel compounds. One particular node, with a molecular weight of 627.3420 Da, caught the researchers’ attention. This compound corresponded to a previously identified amide alkaloid dimer from P. longum, piperchabamide H (m/z 627.3428 [M + H]+). This discovery became the “seed molecule” from which the team isolated 12 dimeric amide alkaloids.
By using chiral high-performance liquid chromatography (HPLC), the team was able to separate these 12 compounds into 12 pairs of enantiomers—molecules that are mirror images of each other. This was an essential step in fully elucidating their structures and understanding their biological activity. The researchers utilized a combination of advanced spectroscopic techniques, including nuclear magnetic resonance (NMR), electronic circular dichroism (ECD) calculations, and X-ray diffraction analysis, to confirm the structures of these alkaloids.
The dimeric amide alkaloids they identified included eight pairs of cyclobutane-type dimers and four pairs of cyclohexene-type dimers. Among these, five pairs of the cyclobutane-type dimers were newly discovered, as well as one pair of a new cyclohexene-type dimer. These compounds are unique, as the chemical structures of cyclobutane and cyclohexene are relatively rare in natural product chemistry, making them of particular interest for further study.
Bioactivity Screening: Anti-Inflammatory and Antidiabetic Effects
With the structures of the alkaloids fully elucidated, the researchers next focused on exploring their biological activities. In vitro bioactivity assays were conducted to evaluate the compounds’ potential therapeutic effects. One of the most striking findings was that three of the isolated compounds exhibited significant anti-inflammatory activity in an LPS-induced RAW 264.7 macrophage model, a standard test for inflammation. Inflammation is a key driver of many chronic diseases, including autoimmune disorders, heart disease, and diabetes, so these compounds could have substantial therapeutic value for managing inflammatory conditions.
Furthermore, one of the compounds demonstrated potent inhibitory activity against α-glucosidase, an enzyme responsible for breaking down carbohydrates into glucose in the intestines. Inhibition of α-glucosidase is an established strategy for managing type 2 diabetes, as it helps control blood sugar levels after meals. Additionally, three other compounds showed promising inhibitory activity against protein tyrosine phosphatase-1B (PTP1B), a key regulator of insulin signaling. PTP1B inhibitors are being explored as potential treatments for diabetes, as they can improve insulin sensitivity and promote glucose homeostasis.
Molecular Docking and Dynamics Simulations: Understanding How These Compounds Work
To delve deeper into how these dimeric amide alkaloids interact with their target enzymes, the research team employed molecular docking studies. These studies simulate how the compounds bind to the active sites of enzymes, providing valuable insights into their mechanisms of action. The molecular docking results revealed that the two most active compounds formed stable complexes with their respective target proteins—α-glucosidase and PTP1B. These findings support the hypothesis that these compounds could be effective inhibitors of these enzymes.
Further molecular dynamics simulations were conducted to assess the stability of these protein-ligand complexes over time. The simulations confirmed that the complexes remained stable, with the compounds binding tightly to the active sites of the enzymes, suggesting that they could effectively block enzyme activity in a biological setting. These results are crucial for understanding the therapeutic potential of these compounds and could guide future drug development efforts.
Implications for Functional Foods and Pharmaceutical Applications
The findings of this study have significant implications for both the pharmaceutical and functional food industries. The identification of these novel dimeric amide alkaloids with anti-inflammatory and antidiabetic effects paves the way for developing new natural products that could be used to manage chronic diseases. Given the growing interest in plant-based therapies and functional foods, these compounds could form the basis for the development of new functional ingredients or supplements aimed at improving metabolic health and reducing inflammation.
Furthermore, the study highlights the potential of P. longum fruits as a source of bioactive compounds for pharmaceutical research. The unique chemical structures of the dimeric alkaloids identified in this study make them promising candidates for further investigation, and their dual anti-inflammatory and antidiabetic effects could position them as lead compounds in drug discovery programs targeting these conditions.
Conclusion: The Future of Piper longum in Modern Medicine
The research conducted by Prof. Haji Akber Aisa and his team represents a significant step forward in understanding the therapeutic potential of P. longum fruits. By employing advanced techniques such as molecular network-based dereplication, the researchers have uncovered a novel class of bioactive compounds that hold promise for the development of new treatments for inflammatory diseases and diabetes.
As the scientific community continues to explore the wealth of natural products found in plants like P. longum, this study serves as a reminder of the untapped potential of traditional remedies. The integration of cutting-edge technologies with the rich heritage of traditional medicine offers a promising avenue for discovering novel therapeutic agents that could benefit patients around the world. The future of P. longum and its bioactive compounds in modern medicine looks bright, with continued research likely to unlock even more of its healing secrets.
Reference: Guanghui Gou et al, Dimeric Amide Alkaloid Enantiomers from Piper longum L. with Anti-Inflammatory and Antidiabetic Effects, Journal of Agricultural and Food Chemistry (2025). DOI: 10.1021/acs.jafc.4c13133