When the world was plunged into the uncertainty of the COVID-19 pandemic, scientists raced against time to find solutions. Amid this whirlwind, a team at UC San Francisco (UCSF) and the Gladstone Institutes set their sights not just on the current pandemic but on safeguarding humanity against future viral threats.
Now, after three years of tireless work, their efforts have culminated in a groundbreaking achievement: the development of new drug candidates that outshine Paxlovid—currently the leading antiviral treatment for COVID-19—not only against SARS-CoV-2 but also against MERS, a deadly coronavirus that continues to cause outbreaks across the globe.
The implications are profound. These compounds may not just address today’s crisis but could form a powerful defense line against the next pandemic.
“In three years, we’ve moved as fast as a pharmaceutical company would have, from start to finish, developing drug candidates against a totally new pathogen,” said Dr. Charles Craik, UCSF professor of pharmaceutical chemistry and one of the leading minds behind the discovery. “These compounds could inhibit coronaviruses in general, giving us a head start against the next pandemic.”
A Race Against Time—and Funding
The journey to this breakthrough was fueled initially by a special grant from the National Institute of Allergy and Infectious Diseases (NIAID), part of a broader effort to bolster the nation’s pandemic preparedness. However, despite the promising results, the grant has since been terminated by the National Institutes of Health (NIH), leaving the future of these antiviral candidates uncertain.
Pharmaceutical companies, largely driven by short-term profit margins, have abandoned much of this critical research, making academic institutions like UCSF vital hubs of innovation. Yet, without ongoing support, even the most promising drug discoveries can languish, never reaching the people who need them most.
From Virtual Simulations to Real-World Solutions
The seeds of this discovery were sown in the earliest days of the pandemic. Recognizing the unprecedented threat COVID-19 posed, Dr. Nevan Krogan, director of the UCSF Quantitative Biosciences Institute (QBI), launched the Coronavirus Research Group (QCRG) in 2020. What followed was a remarkable feat of scientific collaboration: a network of over 800 scientists from 40 institutions worldwide, spanning continents and disciplines, united by a single goal—to defeat the virus.
Krogan assembled teams from UCSF, the Gladstone Institutes, and prestigious institutions like the Icahn School of Medicine at Mount Sinai, Northwestern University, MIT, University of Toronto, and the historic Institut Pasteur, among many others. Their collective expertise was the engine behind the swift progress.
“COVID was our wake-up call,” said Krogan. “It forced us to apply all our resources and know-how towards developing new therapies—not just for this pandemic, but to prepare for those that might follow.”
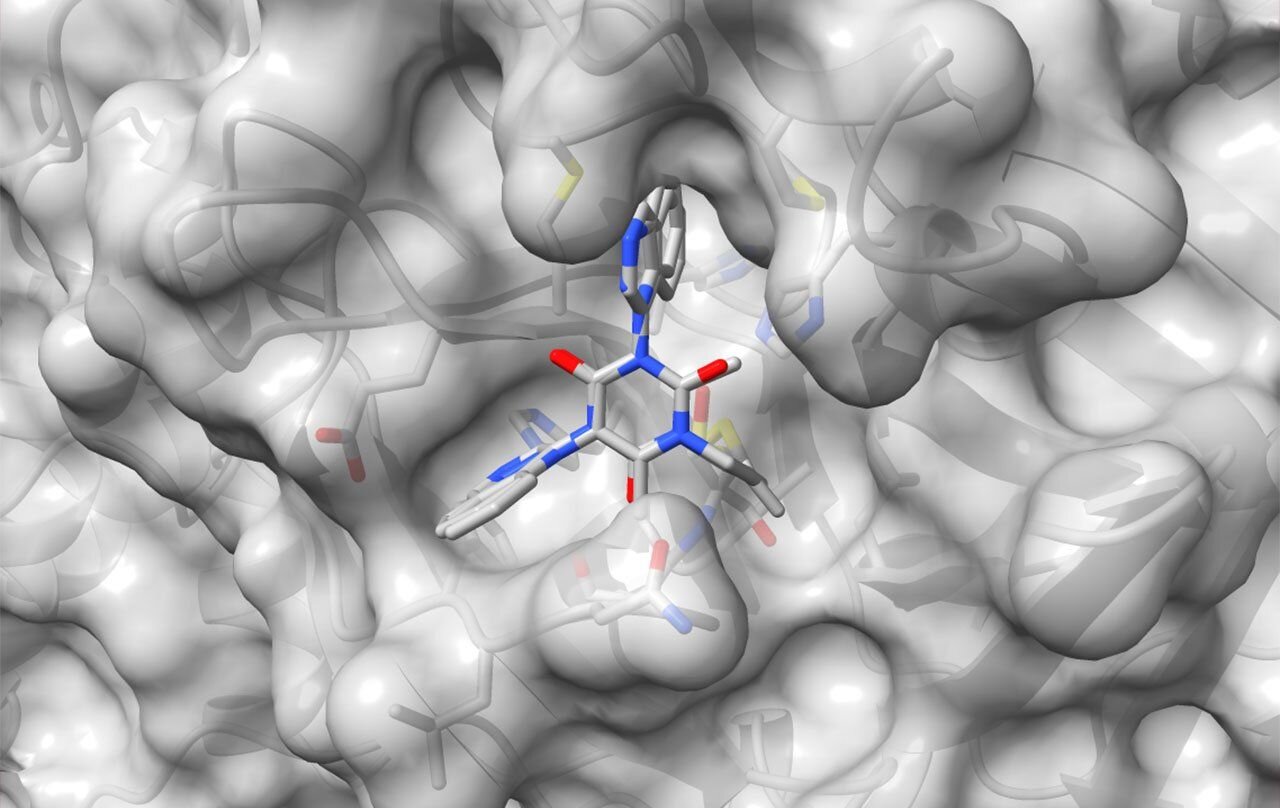
Targeting the Viral Machinery: The Power of MPro
The team honed in on a critical vulnerability in the coronavirus life cycle: the major protease (MPro). This enzyme acts like molecular scissors, slicing long viral proteins into functional pieces that the virus needs to replicate inside human cells. Inhibiting MPro could effectively halt the virus in its tracks, making it an ideal drug target.
Dr. Brian Shoichet’s lab at UCSF played a pivotal role in the first phase. Using advanced molecular docking simulations, they virtually screened millions of compounds, looking for structures that could bind to and disable MPro. Out of this massive search, a few dozen promising candidates emerged.
Taking these initial hits, Dr. Adam Renslo’s lab synthesized hundreds of new molecules, each carefully tailored and refined. Dr. Charles Craik’s team then put these compounds to the test in real-world assays, identifying two standout molecules: AVI-4516 and AVI-4773.
These molecules bonded precisely to the MPro active site—the viral Achilles’ heel—while leaving human proteases untouched. This selectivity promised not only effectiveness but also minimal side effects, a crucial feature for any drug.
“This was our lucky break,” said Craik. “We found molecules that only react once inside the viral protease, sparing our own cells’ crucial enzymes.”
Breaking New Ground in Antiviral Efficacy
Having confirmed the molecular precision of their candidates, the team moved to the next critical step: testing against live virus. This phase fell to Dr. Melanie Ott, a renowned virologist at Gladstone Institutes, who had by then tested hundreds of compounds against SARS-CoV-2.
The results were electrifying. AVI-4516 and AVI-4773 demonstrated unprecedented potency, obliterating the virus in cultured cells and in animal models. In side-by-side comparisons, these new compounds outperformed Paxlovid.
“It’s very challenging to fight viruses in general, let alone SARS-CoV-2,” said Ott. “But these new compounds were some of the best—if not the best—we had ever seen.”
Not only did the drugs powerfully inhibit the virus, but they also showed excellent pharmacokinetics: they traveled through the body efficiently, reached infected tissues, and, critically, appeared safe in animal studies.
The triumph didn’t stop there. Further refinements to the molecules expanded their activity spectrum. The optimized compounds neutralized not only SARS-CoV-2 variants like Delta but also the MERS coronavirus, which is considered a prime candidate for the next coronavirus pandemic.
The Promise—and Peril—of Preparedness
The vision for these drug candidates is compelling: create a “shelf-stable” antiviral, ready to deploy at the first sign of a new coronavirus threat. In a world still grappling with the aftermath of COVID-19, having potent, broad-spectrum antivirals at the ready could save millions of lives and prevent economic devastation.
“This isn’t just about this pandemic,” emphasized Renslo. “This is about being ready for the next one—and the one after that.”
The chemical properties of AVI-4516 and AVI-4773 make them relatively easy to modify and straightforward to manufacture—two huge advantages when speed and scale are crucial in a pandemic response.
However, despite the incredible potential, the program now faces an uncertain path forward. Without renewed funding and support, the drug candidates might remain stuck in preclinical stages, never reaching the clinical trials needed to bring them to patients.
“It’s critical that we see this project through,” Renslo added. “The work we’ve done shows that academic teams, with enough support, can deliver real solutions to global crises.”
Reimagining the Future of Pandemic Response
The UCSF and Gladstone team’s success underscores a broader lesson: the need to invest in proactive pandemic preparedness, not just reactive crisis management. The pandemic revealed glaring gaps in the world’s ability to respond to novel threats. Academic research groups—agile, innovative, and mission-driven—have proven they can fill those gaps if given the resources and autonomy.
The story of AVI-4516 and AVI-4773 offers a glimpse into a more hopeful future, one where humanity does not stand helpless in the face of emerging viruses. Instead, it envisions a future where science, collaboration, and foresight converge to create a robust shield against the microbial threats that have shaped our history and will continue to challenge us.
Conclusion: A Critical Crossroads
Standing at this crossroads, the question is no longer whether the UCSF and Gladstone team can create life-saving antivirals—they already have. The question is whether society will have the vision and commitment to nurture these breakthroughs, to usher them through clinical development, and to ensure they are ready when the next viral storm breaks.
It’s a reminder that while viruses evolve rapidly, so too can human ingenuity—if we choose to invest in it.
In the race against pandemics, the clock is always ticking. Thanks to the dedicated work of these scientists, we now have a head start. What remains to be seen is whether we will finish the race in time.
Reference: Tyler C. Detomasi et al, Structure-based discovery of highly bioavailable, covalent, broad-spectrum coronavirus M Pro inhibitors with potent in vivo efficacy, Science Advances (2025). DOI: 10.1126/sciadv.adt7836
Loved this? Help us spread the word and support independent science! Share now.