Imagine a world where objects can exist in two places at once, where particles can be “entangled” across vast distances, and where the future of the universe is fundamentally uncertain. This world is not one of science fiction—it is the strange and baffling realm of quantum mechanics, the branch of physics that describes the behavior of matter and energy on the smallest scales, such as atoms and subatomic particles.
For over a century, quantum mechanics has been the foundation of much of modern physics, revolutionizing our understanding of the universe. From the atoms that make up the matter around us to the very nature of light and time itself, quantum mechanics offers a profound and puzzling explanation for the workings of the universe.
But quantum mechanics isn’t just a theoretical framework confined to the pages of physics textbooks. It’s a field that has practical implications and has already revolutionized technology, medicine, and communication. The digital age, for example, is built on principles derived from quantum mechanics, as is much of the cutting-edge research in computing, telecommunications, and even artificial intelligence.
In this article, we’ll explore what quantum mechanics is, how it emerged, and how its fundamental principles shape the world we live in. We’ll dive deep into the paradoxes and mysteries of quantum physics and show you how these abstract ideas translate into real-world applications that are changing our lives.
The Birth of Quantum Mechanics
To understand quantum mechanics, we first need to take a step back in time to the early 20th century. Classical physics, based on the work of Isaac Newton, had long been the dominant framework for understanding the universe. It worked wonderfully for most everyday phenomena: the motion of planets, the behavior of gases, and even the workings of machines. However, as scientists began to probe deeper into the microscopic world of atoms and subatomic particles, they encountered phenomena that classical physics simply couldn’t explain.
The story of quantum mechanics begins with the mysterious behavior of light. In the late 19th century, scientists had established that light behaves as both a wave and a particle. But this dual nature of light raised troubling questions. For instance, the “photoelectric effect,” discovered by Heinrich Hertz and later explained by Albert Einstein in 1905, showed that when light shines on a metal surface, it can knock electrons off the metal. This phenomenon seemed to suggest that light behaves like a particle, with discrete packets of energy called “quanta” (later named photons). Einstein’s explanation of the photoelectric effect was a crucial turning point in the development of quantum theory.
Around the same time, Max Planck, a German physicist, was working on the problem of black-body radiation—how objects emit radiation when heated. Classical physics predicted that a hot object should emit infinite energy at high frequencies, but experiments showed this wasn’t the case. In 1900, Planck proposed that energy is emitted in discrete amounts, or “quanta.” This radical idea marked the birth of quantum theory.
Shortly afterward, in 1913, Niels Bohr developed his model of the atom, which incorporated Planck’s ideas. Bohr suggested that electrons orbit the nucleus in specific, quantized energy levels, and could only absorb or emit energy when they jumped between these levels. This was a significant departure from classical physics, which treated energy as continuous.
By the 1920s, quantum mechanics had been fully established. Werner Heisenberg, Erwin Schrödinger, and Paul Dirac each made critical contributions that helped to shape the modern theory. Schrödinger’s wave equation, Heisenberg’s uncertainty principle, and Dirac’s relativistic equations were all key to understanding the bizarre behavior of particles on the quantum scale.
Key Principles of Quantum Mechanics
Quantum mechanics is built on several principles that may seem counterintuitive or even downright bizarre. These principles are:
- Wave-Particle Duality: This concept suggests that matter, like light, can behave both as a wave and as a particle, depending on how it is observed. For example, electrons can behave like waves, creating interference patterns, but when observed, they behave as particles, striking specific locations.
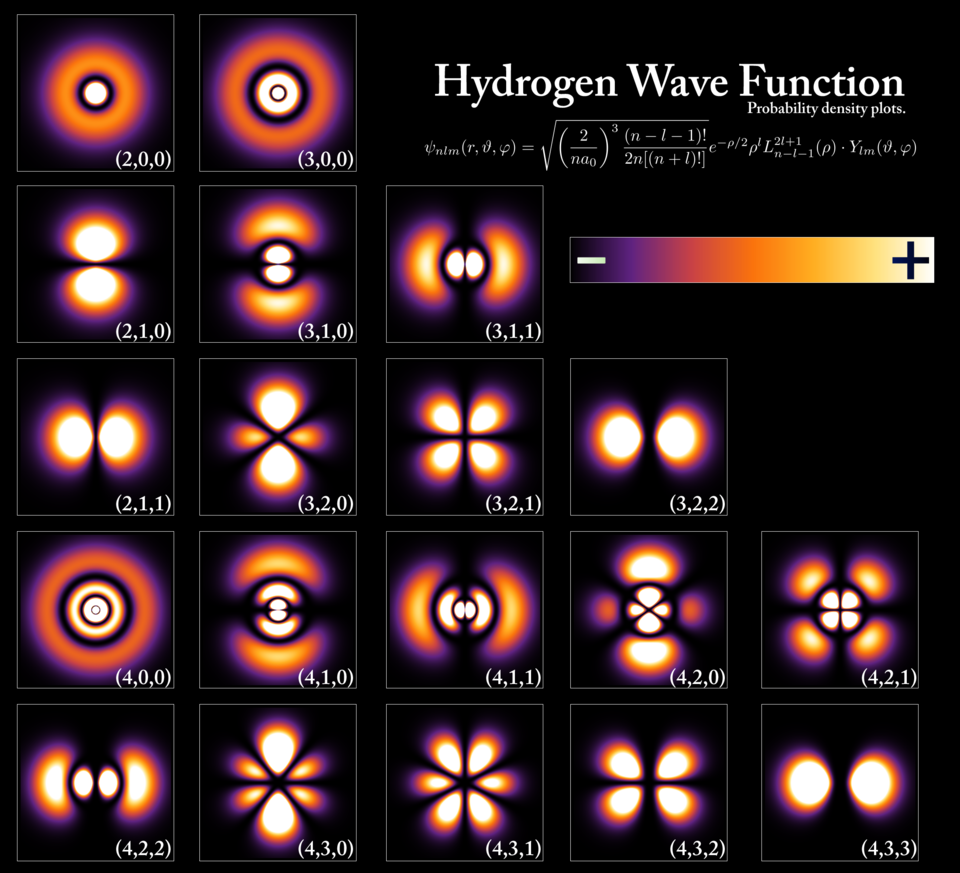
- Superposition: In the quantum world, particles like electrons do not have a single, well-defined state. Instead, they can exist in multiple states at once. This is called superposition. A particle, like an electron, can be in more than one location or energy state at the same time until it is measured. Only when it is measured does it “collapse” into one state.
- Entanglement: Perhaps one of the most mind-bending phenomena of quantum mechanics, entanglement occurs when particles become correlated in such a way that the state of one particle instantly affects the state of another, no matter how far apart they are. This means that if you manipulate one particle, the other will change instantaneously, even if they are light-years apart. This mysterious “spooky action at a distance” perplexed even Einstein, who famously referred to it as “spooky.”
- The Uncertainty Principle: Formulated by Heisenberg, this principle states that it is impossible to simultaneously know both the position and the momentum of a particle with perfect accuracy. The more precisely you measure one quantity, the less precisely you can measure the other. This doesn’t arise from limitations in measurement tools; it is a fundamental property of nature.
- Quantization of Energy: In quantum mechanics, energy levels are quantized, meaning that particles such as electrons can only occupy specific, discrete energy states. They cannot exist in between these states. When an electron jumps from one energy level to another, it either absorbs or emits energy in the form of a photon.
- The Observer Effect: In quantum mechanics, the act of observation can influence the state of the system being observed. This is perhaps most famously illustrated by the double-slit experiment, where particles like electrons behave differently when they are observed compared to when they are unobserved. This raises questions about the role of consciousness in the measurement process and whether the act of observation itself determines the state of reality.
The Double-Slit Experiment: A Quantum Paradox
One of the most famous and thought-provoking experiments in quantum mechanics is the double-slit experiment. In this experiment, particles such as photons or electrons are directed at a barrier with two slits. If the particles behave like classical particles, we would expect them to go through one slit or the other, and form two bands of impact on a detection screen behind the barrier.
However, when the experiment is conducted with individual particles, something remarkable happens. Instead of forming two distinct bands, the particles create an interference pattern, as if they are behaving like waves and passing through both slits simultaneously. This suggests that each particle is in a superposition of states, being in both slits at once. When the experiment is observed, however, the interference pattern disappears, and the particles behave like classical particles.
This experiment illustrates the bizarre and counterintuitive nature of quantum mechanics. It challenges our classical intuition and raises fundamental questions about the nature of reality, observation, and measurement. Does the universe exist in a definite state, or is it only when we observe it that it “decides” what state to be in?
How Quantum Mechanics Affects Our World
Though quantum mechanics describes phenomena on the tiniest scales, its implications are vast and far-reaching. The strange principles of quantum mechanics don’t just stay confined to the microscopic realm—they shape the technology and innovations that define our modern world.
Quantum Technology: From Computers to Cryptography
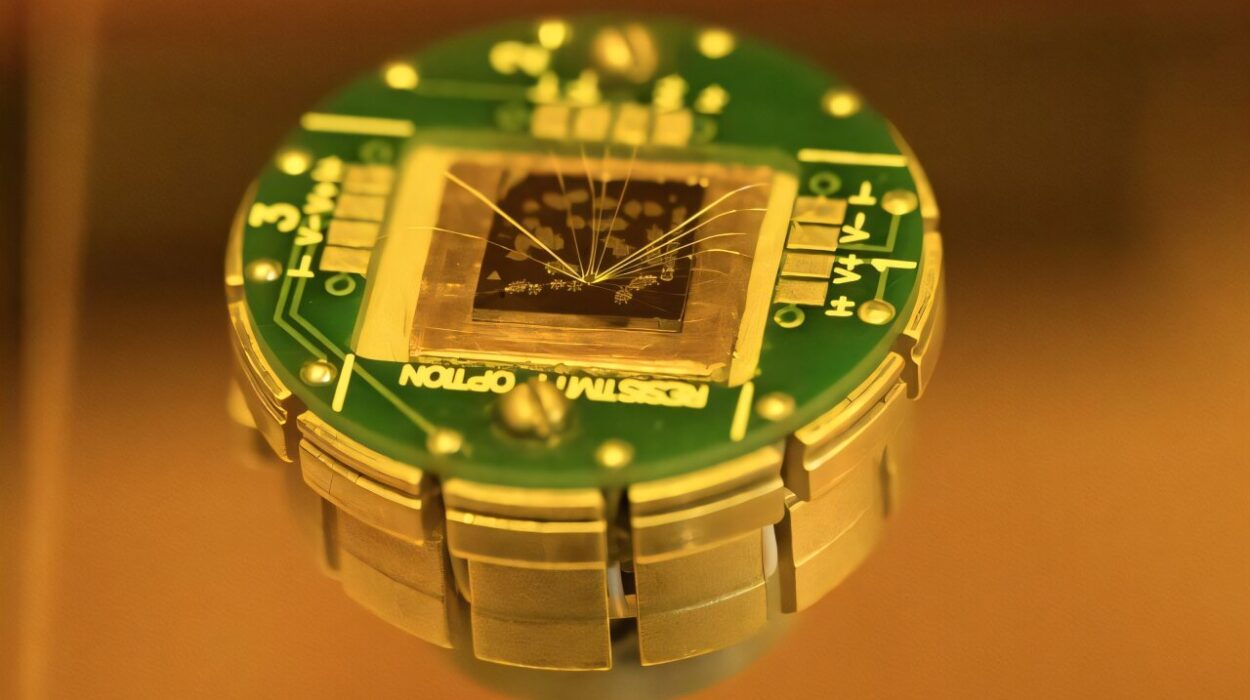
Perhaps the most well-known applications of quantum mechanics are in the fields of computing and telecommunications. The development of quantum computers holds the potential to revolutionize the way we process information. Unlike classical computers, which use bits to represent information as either a 0 or a 1, quantum computers use quantum bits, or qubits, which can exist in multiple states simultaneously, thanks to superposition. This allows quantum computers to perform calculations at speeds far beyond the capabilities of classical computers, solving problems that are currently intractable.
Quantum mechanics also underpins the development of quantum cryptography, a field that promises to provide ultra-secure communication. Quantum key distribution (QKD) takes advantage of the principles of quantum mechanics to exchange encryption keys in a way that makes eavesdropping practically impossible. If an intruder tries to intercept the communication, the quantum state of the system would be disturbed, alerting both parties to the presence of the eavesdropper.
Quantum Sensors and Metrology
Another exciting application of quantum mechanics is in the realm of precision measurement. Quantum sensors, which leverage the unique properties of quantum systems, are being developed to measure time, temperature, magnetic fields, and gravitational forces with unprecedented accuracy. Quantum clocks, for example, are now so precise that they can measure time with an error of less than one second every 30 billion years.
The Future of Quantum Technology: Quantum Internet and More
One of the most ambitious goals of quantum technology is the development of a quantum internet. A quantum internet would use quantum entanglement and quantum key distribution to create a network where data can be transmitted securely and with complete privacy. Such a system could transform communication, making it theoretically impervious to hacking.
Quantum technology could also play a significant role in fields like medicine. Quantum imaging techniques could allow for non-invasive medical scans with incredible precision, potentially revolutionizing diagnostic methods. Quantum biology is another emerging field that explores how quantum phenomena might play a role in biological processes, such as photosynthesis and enzyme reactions.
Conclusion: Embracing the Quantum Future
Quantum mechanics is not just a subject confined to the world of theoretical physics; it is the cornerstone of modern technology and a key player in shaping the future of our world. From quantum computers that could solve problems beyond the reach of today’s supercomputers to secure communication networks that protect our privacy, quantum mechanics is poised to revolutionize the way we live, work, and interact.
Though quantum mechanics remains a deeply puzzling and enigmatic field, its applications continue to expand, offering exciting possibilities for the future. Whether you’re fascinated by the strange paradoxes of quantum theory or excited about the practical innovations it brings, one thing is clear: the quantum world will continue to shape our reality in ways we are only beginning to understand.
As we continue to unlock the mysteries of quantum mechanics, the line between science fiction and science fact becomes ever thinner. The strange, mysterious, and wonderful world of quantum physics is no longer confined to theoretical musings—it is an integral part of our everyday lives, and its impact will only grow in the years to come.