DNA replication is a fundamental and continuous process that occurs in our cells as many as trillions of times a day. Every time a cell divides—whether to heal damaged tissue, replace aging cells, or promote growth—DNA is meticulously copied to ensure that new cells inherit the same genetic blueprint. This delicate, yet crucial process has remained largely elusive to scientists due to its intricate nature, which makes it difficult to observe in real-time.
For years, researchers have struggled to understand how DNA replication occurs with fidelity, primarily because traditional methods were either invasive, damaging, or limited to short stretches of DNA. However, a breakthrough study from the Gladstone Institutes has shed new light on this process. The research team, led by Dr. Vijay Ramani, used a revolutionary new method that combines long-read DNA sequencing with a predictive artificial intelligence (AI) model, offering insights into the elusive moments that follow DNA replication.
The Complexities of DNA Replication
Dr. Vijay Ramani, a leader in single-cell genomics, explained that understanding DNA replication has been difficult due to a fundamental challenge: the replication machinery “destroys” existing DNA structures, creating temporary vulnerability. For newly replicated DNA to be passed on properly to new cells, it needs to re-establish its original structure, but previous research was unable to provide a comprehensive method for observing this phenomenon in action.
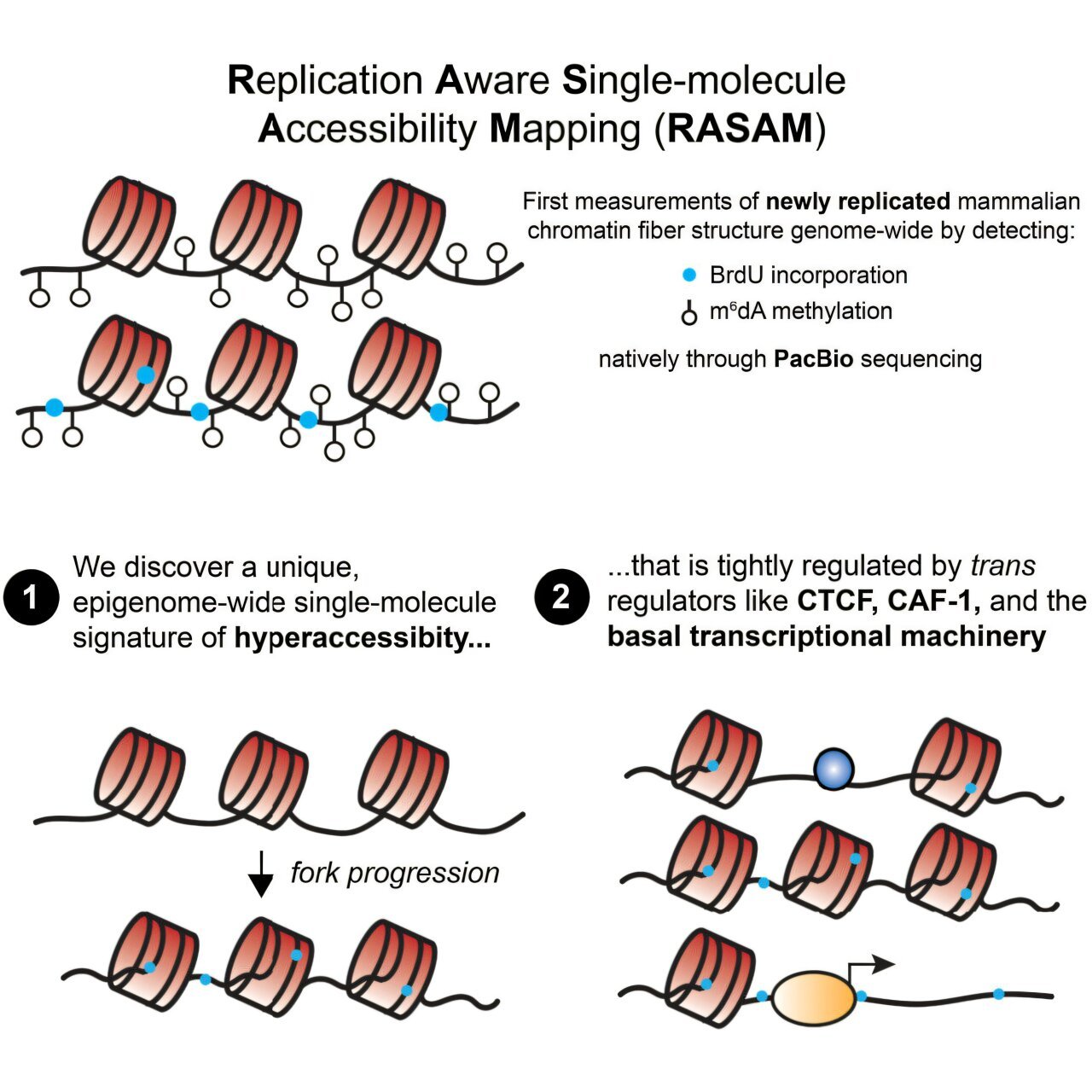
The Gladstone Institute team, which is known for developing innovative techniques in single-cell genomics, needed to devise a new method to answer this longstanding biochemical question. Using RASAM (Replication-Aware Single-Molecule Accessibility Mapping), they successfully investigated the precise changes in DNA accessibility during the critical moments following replication.
Revolutionizing DNA Mapping: The RASAM Method
RASAM, the new technique introduced by the team, has proved invaluable in answering this question. For the first time, this tool allows for a deep, precise understanding of what happens to newly formed DNA in the hours following replication. Traditionally, methods for capturing DNA after replication either damaged the structure of the DNA or failed to observe it on a large enough scale, leaving major gaps in our understanding.
What the Gladstone team discovered was nothing short of revolutionary. The team found that large sections of newly synthesized DNA are hyperaccessible—meaning these DNA regions are particularly open to being accessed by other proteins, including those responsible for regulating gene expression. This hyperaccessibility lasted much longer than previously thought, opening new questions about the control and function of our genetic material in post-replicative states.
According to Dr. Ramani, “We would have thought this level of access would cause genomic haywire, but that’s not what happens.” Instead, it turns out that DNA is partially “unwrapped” right after replication, remaining “loose” or relatively open for hours before becoming fully compacted into nucleosomes (the functional units of DNA packaging). This surprising behavior challenges the established view of how quickly and tightly DNA should wrap itself to protect its integrity.
What Makes Newly Synthesized DNA So Unique?
The idea that nascent (newly formed) DNA remains unusually accessible for extended periods provides fascinating insights. Unlike mature DNA, which is securely packed into nucleosomes, the new study found that newly replicated DNA has an open configuration, providing a unique window during which specific proteins can interact with the genetic material.
In their experiments, Ramani’s team explored how this transient state of “looseness” might provide opportunities for interactions that would otherwise not be possible if the DNA were fully coiled. Surprisingly, this state of accessibility did not lead to chaotic gene expression, as one might expect when DNA is unwrapped or unprotected. Instead, it appeared that this accessible state is carefully regulated and controlled at specific regions along the DNA where gene expression is initiated.
This newly discovered “window of opportunity” in DNA accessibility has profound implications. For one, it may unlock strategies for manipulating gene expression in a precise way, potentially allowing scientists to control specific genetic instructions in cells. This could have valuable therapeutic applications, such as designing treatments that target rapidly dividing cells during this temporary period of DNA vulnerability.
Cancer and the Possibilities for Targeted Medicine
One of the key areas where this finding could make a tremendous impact is in the treatment of cancer, which is characterized by the unchecked and rapid division of cells. Cancer cells divide far more frequently than normal cells, and with these new discoveries about DNA accessibility, researchers may be able to target those cells precisely during their brief, unprotected state post-replication.
Dr. Ramani explains, “By identifying this transient state after replication, it might be possible to design medicines that directly interfere with these vulnerable moments, potentially stopping cancer cells before they can divide again. This could open up an entirely new avenue for anti-cancer treatments.”
Though this possibility is still being explored, it shows the power of precise DNA mapping and real-time observation in tackling complex diseases. By pinpointing the DNA’s more “accessible” state, scientists could time treatments to target these phases effectively, maximizing their effectiveness and minimizing potential harm to normal, healthy cells.
DNA Accessibility and Gene Regulation: New Insights into Health and Disease
Beyond cancer, the RASAM method is also helping to map out previously unseen regions of the genome. By using this cutting-edge method, Ramani’s team was able to observe the intricate regulation of gene expression at locations where the DNA molecule “unwinds” just enough to allow essential proteins access to the genetic code. These proteins often play pivotal roles in transcription, which is the first step in translating DNA into proteins.
In their research, the scientists showed that these regulatory sites where DNA “loosens” are incredibly crucial for cellular processes, which are in turn linked to the function and dysfunction of genes. Gene expression can be influenced by many factors, including the state of DNA packaging, its accessibility to proteins, and the cell’s environment. With RASAM, we can now begin to uncover just how these processes unfold at the molecular level, offering insights into what might go wrong in diseases like genetic disorders, aging, and neurodegenerative diseases.
Moreover, the scientists found that certain proteins that regulate gene expression preferentially bind to these newly accessible regions after replication, helping guide how the DNA regains its functional structure. For scientists, understanding this mechanism opens up exciting new areas for investigation, including how to manipulate gene expression in specific ways to correct or prevent certain diseases.
Future Research Directions: Unanswered Questions and the Power of New Technology
While the breakthroughs presented in the study are groundbreaking, several questions remain unresolved. For example, scientists are curious about how newly formed cells avoid damage or unwanted mutations during the period of DNA accessibility and whether any additional processes come into play to maintain the integrity of the replicated genetic material.
Additionally, Dr. Ramani notes, “What I love about this work is that it’s all about the methods that enable discovery.” The ability to track genomic dynamics in unprecedented detail could help clarify additional mysteries within cell biology. As the technology progresses, scientists will be able to look even deeper into the genomic processes at a molecular and cellular level. This presents opportunities to further understand fundamental biological processes and their connections to disease, potentially leading to more targeted and effective medical treatments.
Dr. Ramani’s research shows that as we enhance the precision of our biological tools, we move closer to transforming basic understanding into practical treatments. By creating methods like RASAM, researchers open doors to previously impossible levels of observation, expanding our knowledge of the most complex inner workings of the genome.
The research team at Gladstone Institutes, with their novel approach, not only highlights the mysteries of DNA replication but also reaffirms the power of new technologies in shaping the future of medicine. As they continue to explore the fascinating processes involved in DNA and gene regulation, the discoveries made here could very well redefine how we approach the treatment of disease and our fundamental understanding of the human genome.
Conclusion
The groundbreaking research from the Gladstone Institutes, utilizing the RASAM method, provides unprecedented insight into the dynamics of DNA replication and its aftermath. By revealing that newly synthesized DNA remains highly accessible for hours after replication, the study challenges long-standing assumptions about DNA packaging and opens new possibilities in gene regulation and disease treatment. Mapping this transient “loose” state of DNA presents exciting opportunities for therapeutic development, especially in fields like cancer treatment, where targeting vulnerable cells during rapid division could lead to more effective therapies. This innovative approach also enhances our understanding of fundamental cellular processes, facilitating further exploration of how genetic material is regulated and protected after replication. As this technology advances, it holds the potential to revolutionize not only our understanding of biology but also the way we approach medicine and disease prevention.
Reference: Megan S. Ostrowski et al, The single-molecule accessibility landscape of newly replicated mammalian chromatin, Cell (2024). DOI: 10.1016/j.cell.2024.10.039
Think this is important? Spread the knowledge! Share now.