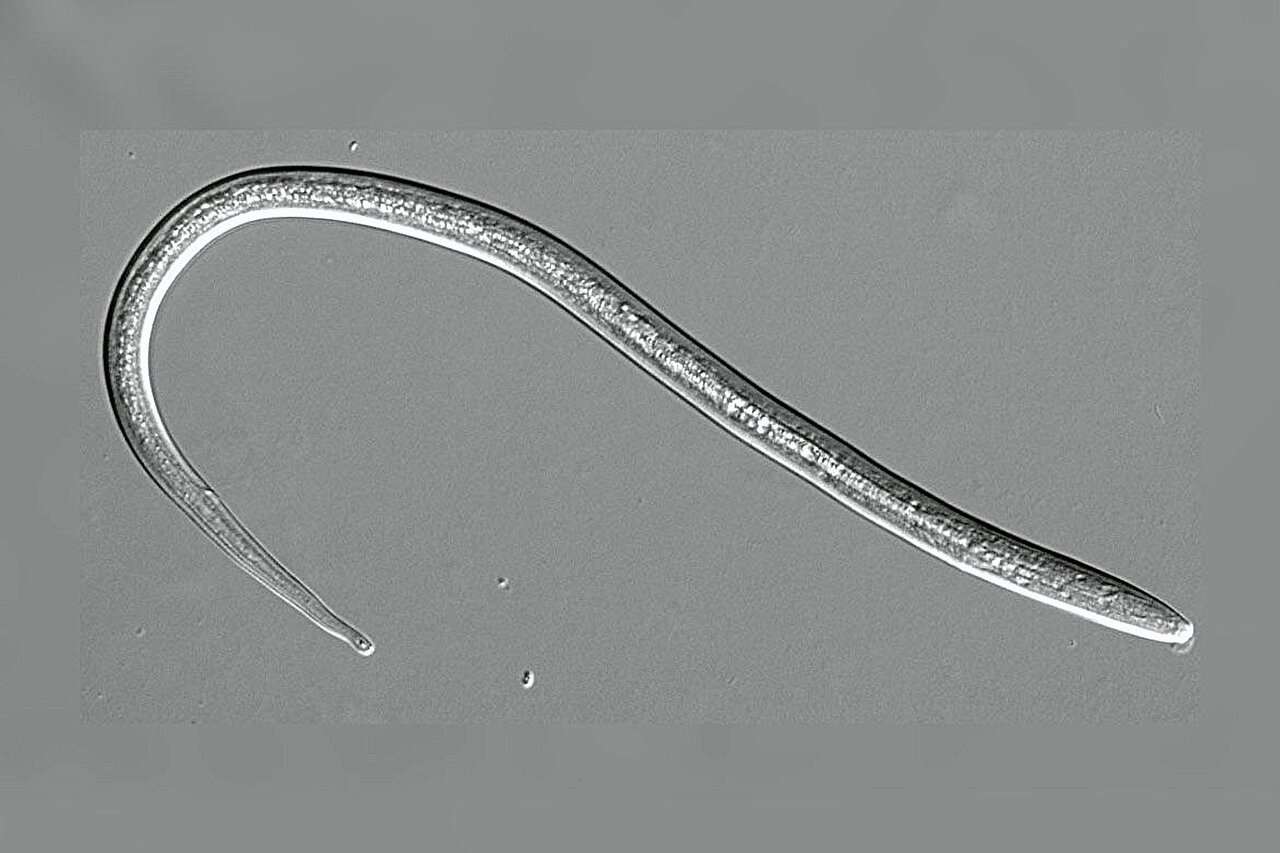
In the United States, hookworms are commonly recognized as skin-penetrating nematodes responsible for parasitic infections. However, globally, another, lesser-known nematode—Strongyloides stercoralis—infects over 600 million people, causing a major public health concern in tropical and subtropical regions with inadequate sanitation infrastructure. This parasitic worm, also called the threadworm, is transmitted when larvae exit the feces of infected hosts, burrowing through the skin of a new host. Despite current treatments, the rise of drug resistance necessitates the development of new therapies, and researchers at the University of California, Los Angeles (UCLA), have uncovered a potential avenue for future treatments.
Global Burden of Skin-Penetrating Threadworm Infections
Threadworm infections are highly prevalent in impoverished areas where sanitation is poor. These skin-penetrating nematodes infect the human host through direct contact with contaminated soil. When larvae hatch from the eggs excreted by infected individuals, they seek new hosts to initiate infection. The nematode larvae penetrate the skin, travel through the body—often passing through organs such as the lungs—and settle in the small intestine, where they reproduce and lay eggs that are then excreted in feces. This life cycle of infection continues to perpetuate the transmission of the disease.
In the most severe cases, these infections can cause serious health complications, such as intestinal disorders, malabsorption of nutrients, respiratory distress, and even death in immunocompromised individuals. The global distribution of S. stercoralis spans tropical and subtropical areas, but it is not confined solely to impoverished nations. It is capable of circulating in areas where inadequate sanitation and sewage infrastructure allow fecal contamination to persist in soil. Despite the availability of treatments like ivermectin, which is effective against many parasitic nematodes, some strains of S. stercoralis are becoming resistant to this drug, highlighting the need for new therapeutic approaches.
UCLA Researchers’ Breakthrough on CO2-Sensing in Threadworms
One promising development in the fight against parasitic nematode infections comes from a team of neurobiologists at UCLA. Their research, published in Current Biology, investigates a novel aspect of S. stercoralis’s biology: the parasite’s response to carbon dioxide (CO2) at different stages in its life cycle.
The discovery that these threadworms respond to CO2 differently at each stage of their life could prove to be a crucial stepping stone in the search for new treatments. CO2 is a ubiquitous gas found in many tissues of the human body, including the lungs and intestines. It is also naturally present in the fecal environment and surrounding soil, where the infective larvae initially seek a new host.
Elissa Hallem, a professor of microbiology, immunology, and molecular genetics at UCLA and corresponding author of the study, explains that carbon dioxide plays a vital role in the interactions between these parasitic nematodes and their human hosts as the worms progress through different stages of their life cycle. These reactions to CO2 exposure could be leveraged in therapeutic design, offering a way to manipulate or block the sensory mechanisms that help the worms navigate their host environments.
The Threadworm Life Cycle and the Role of CO2
The first stage of a Strongyloides stercoralis infection begins when larvae are excreted in the feces of an infected host. These immature larvae develop into infective larvae in the surrounding environment, often in moist soil, where they seek out new hosts. Once a suitable host is found, larvae burrow into the host’s skin, ultimately reaching the intestines, where they establish themselves as adult parasitic worms. They then lay eggs that are released in the host’s stool, continuing the life cycle.
The research team at UCLA observed that CO2 concentration influences the movement of these nematodes during their cycle. Infective larvae—the stage responsible for initiating infection—show a marked aversion to CO2, while noninfective larvae and adult worms appear unaffected by changes in CO2 levels. However, once the worms enter the body of the host, the dynamics change. Young migrating worms inside the host are actually attracted to CO2, which might guide them toward critical organs, including the lungs and intestines.
The Mechanism Behind CO2-Sensing: Neurons and Genetic Pathways
The research team took a deeper look at the sensory system that facilitates this unique behavioral response. They focused on two key discoveries: specific neurons that are responsible for detecting CO2 and a particular gene, called GCY-9, that codes for a receptor helping the worms sense this gas. By manipulating the gene responsible for GCY-9 in the laboratory, they observed a loss of CO2-sensing behavior in the nematodes, confirming that GCY-9 is integral to the CO2 response mechanism. This discovery is vital because it opens the door to designing therapeutic interventions that target the CO2-sensing pathway, potentially interfering with the parasite’s ability to establish infection.
According to lead author and postdoctoral researcher Navonil Banerjee, disrupting this CO2-sensing mechanism could impair the threadworm’s ability to navigate its environment. In particular, by blocking CO2 detection, drugs could prevent infective larvae from migrating into the host’s body, thus stopping the transmission cycle in its tracks or reducing the severity of infections once they have occurred.
Implications for Drug Development
The findings from this study could be crucial in developing new drugs that specifically interfere with the threadworm’s ability to detect CO2, one of the critical sensory cues that guide the parasite through the host body. Disrupting the nematode’s ability to track CO2 could impair its life cycle, reduce the effectiveness of its internal migration, and potentially prevent the worm from establishing the parasitic infection.
Future studies will likely expand on these findings to explore other genes and molecular targets involved in CO2 detection and navigation within the host. By identifying additional molecules responsible for chemoreception or influencing parasitic behavior, researchers could further refine new therapeutic strategies aimed at disrupting the parasitic nematodes’ ability to infect human hosts.
Potential for Broader Applications
Though the research focused on S. stercoralis, understanding how parasitic nematodes sense environmental cues like CO2 could have broader applications. Many other species of parasitic nematodes rely on similar sensory mechanisms to locate suitable hosts. By discovering shared pathways across different species, researchers could create drugs capable of targeting multiple parasites simultaneously, improving the overall efficacy of treatments for skin-penetrating nematodes.
Moreover, the exploration of the role of CO2 and other environmental sensory cues in parasitic life cycles could drive future innovations in both public health strategies and pharmaceutical design. More targeted therapies would enhance the efficiency of existing treatments, potentially overcoming current drug resistance issues and minimizing the side effects often associated with broad-spectrum antiparasitic drugs.
Conclusion
The study from UCLA represents a significant leap in understanding the behavioral and sensory biology of parasitic nematodes such as Strongyloides stercoralis. Their discovery of the CO2-sensing pathway is a promising development that could lead to new, more effective treatments for parasitic infections, especially as drug resistance continues to increase. By leveraging these molecular insights, researchers may soon have new ways to combat some of the most dangerous and persistent parasitic diseases affecting millions of people worldwide. These advancements not only offer hope for the future of treating parasitic infections but also highlight the power of basic scientific research to address complex global health challenges.
Reference: Navonil Banerjee et al, Carbon dioxide shapes parasite-host interactions in a human-infective nematode, Current Biology (2024). DOI: 10.1016/j.cub.2024.11.036