In the ever-escalating battle between humanity and infectious disease, scientists have just unveiled a potential game-changer. A coalition of French researchers—drawn from INRAE, CNRS, Université Paris-Saclay, and Inserm—has identified a small molecule that can subtly “disarm” pathogenic bacteria without disturbing the beneficial microbes that dwell peacefully within our bodies. This discovery, recently published in Nature Communications and already protected by patents, may signal the dawn of a revolutionary approach to antibiotic therapy—one that could outmaneuver the escalating threat of antibiotic resistance.
Antibiotic Resistance: A Crisis of Our Own Making
When antibiotics first emerged in the 20th century, they were hailed as miracle drugs. Diseases that once killed millions—tuberculosis, syphilis, pneumonia—were suddenly treatable, often within days. But the euphoria of those early decades led to complacency. Antibiotics were prescribed liberally and sometimes unnecessarily, not just in medicine but also across agriculture and animal husbandry.
The consequence? A relentless rise in antibiotic resistance.
According to the World Health Organization, antibiotic-resistant infections are now responsible for at least five million deaths globally each year. If left unchecked, this figure could balloon to surpass cancer as the leading cause of death by 2050. As bacteria evolve and mutate, our once-powerful drugs become ineffective. Worse still, the development pipeline for new antibiotics has slowed to a trickle.
What we need is not just new antibiotics—but smarter ones.
The Bacterial Achilles’ Heel: A Hidden Protein Called Mfd
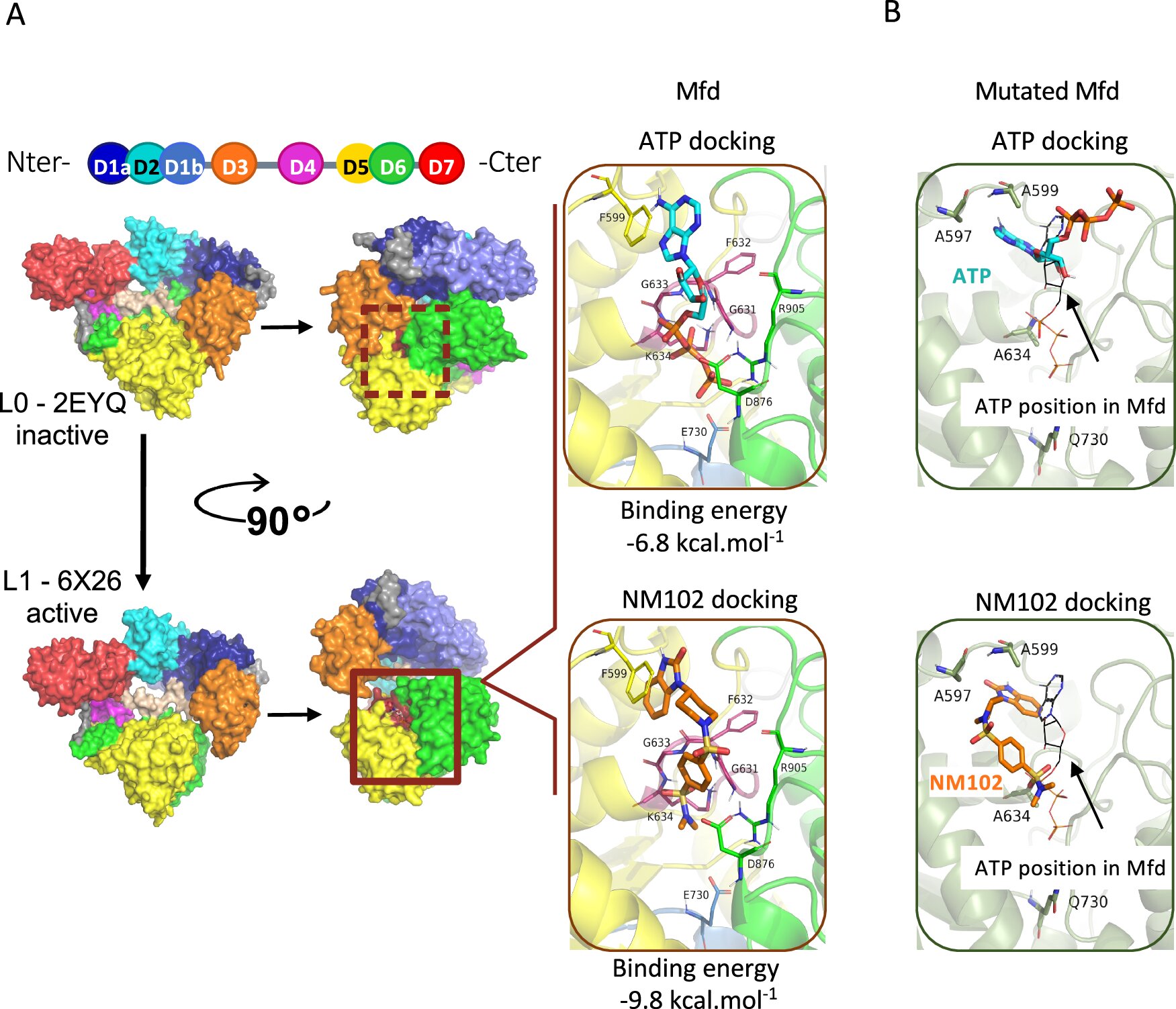
Enter the Mutation Frequency Decline (Mfd) protein—a molecular multitasker lurking within all bacteria. Previously overlooked, Mfd has now stepped into the spotlight thanks to an ingenious line of inquiry from INRAE’s research team.
Mfd performs two critical roles: First, it allows bacteria to dodge the immune system by enhancing their ability to survive oxidative bursts—lethal chemical attacks launched by immune cells. Second, and perhaps more disturbingly, it accelerates genetic mutations within bacterial DNA. These mutations, largely random, give rise to traits like antibiotic resistance.
Imagine a bacterial mastermind—one that doesn’t kill, but instead teaches its troops to adapt, evolve, and strike back harder. That’s Mfd.
Targeting the Enemy Without Collateral Damage
In a field where traditional antibiotics act like sledgehammers—killing everything, including friendly bacteria—the search for precision weapons has become urgent. Broad-spectrum antibiotics, while effective in wiping out pathogens, also obliterate parts of the microbiota, the diverse community of microbes that plays essential roles in digestion, immunity, and even brain health.
The innovation introduced by the INRAE-led consortium avoids this trap.
Instead of seeking to kill bacteria outright, the scientists sought a subtler strategy: disabling the bacteria’s ability to resist the immune system and evolve defenses. In short, they set out to neutralize Mfd.
To do this, they tapped into a colossal molecular library of over five million compounds. After exhaustive screening, a standout candidate emerged—NM102, a small molecule that binds directly to Mfd and prevents it from switching into action.
What makes this finding especially compelling is its specificity. NM102 doesn’t kill bacteria outright. It waits, allowing the immune system to attack as it normally would. But now, with Mfd silenced, the bacteria are vulnerable. They can’t dodge the immune response, nor can they mutate their way to resistance. They’re essentially sitting ducks.
NM102 in Action: Laboratory Proof and Beyond
To put their theory to the test, researchers turned to both in vitro studies and live models. The results were startling.
In insect and mouse models infected with pathogenic bacteria, NM102 showed a trifecta of beneficial effects:
- First, it didn’t harm bacteria in sterile environments. This indicated that NM102 wasn’t bactericidal—it didn’t kill on its own, minimizing the likelihood of resistance through selective pressure.
- Second, in the presence of an active immune system, it significantly reduced bacterial load in infected organs, meaning it gave the host the upper hand in fighting the infection.
- Third, and most importantly, it disrupted the mutagenic function of Mfd. With Mfd off the table, the bacteria lost their capacity to develop resistance to antibiotics—even under drug pressure.
In a world plagued by superbugs, this is nothing short of a scientific masterstroke.
Preserving the Peacekeepers: Protecting the Microbiome
One of the greatest strengths of NM102 is what it doesn’t do. Traditional antibiotics act indiscriminately, wiping out friendly and harmful bacteria alike. The resulting imbalance, or dysbiosis, can trigger secondary infections, digestive issues, and even contribute to chronic diseases.
But NM102 leaves the microbiota untouched.
Because it acts only in the presence of immune stress and doesn’t kill directly, beneficial bacteria remain unscathed. In fact, the compound helps restore a critical balance: letting our natural defenses do the job while ensuring that only the troublemakers are disarmed.
This selectivity could redefine how we think about antimicrobial therapy. Instead of eradication, the future may lie in coexistence—where pathogens are disarmed rather than destroyed, and the delicate architecture of the human microbiome is preserved.
The Road to Drug Development
With two patents already filed—one for the identification of Mfd as a target, and the other for the molecule itself—the race is now on to turn NM102 from a laboratory discovery into a real-world drug.
Researchers are working with the Commissariat à l’Énergie Atomique et aux Énergies Alternatives (CEA) to optimize the molecule’s structure. In parallel, they’ve successfully encapsulated NM102 in biodegradable nanoparticles, paving the way for controlled, targeted delivery within the body.
This step is vital. Drug candidates often fail not because they’re ineffective, but because they can’t reach the right place, in the right amount, at the right time. Encapsulation ensures that NM102 can be administered safely and precisely, minimizing off-target effects.
Clinical trials are still a few years away, but the groundwork is promising. If successful, NM102-based therapies could enter the antimicrobial arsenal as a new class of precision antibiotics—one that sidesteps the pitfalls of resistance and collateral microbiome damage.
Implications for Global Health
The implications of this discovery stretch far beyond the lab.
Hospitals, currently ground zero for antibiotic-resistant infections, could deploy NM102-derived drugs to treat patients harboring resistant strains. Surgeons and oncologists—whose patients are especially vulnerable to infection—might one day use such therapies prophylactically, boosting immune efficiency without disrupting the microbiome.
Public health campaigns could finally pivot from “kill the germs” to “disarm the invaders,” introducing a new vocabulary for infection management that reflects biological nuance rather than brute force.
In resource-limited settings, where access to expensive new antibiotics is often out of reach, NM102-like molecules may offer an affordable, scalable way to prolong the usefulness of existing drugs.
The Bigger Picture: A Shift in Therapeutic Philosophy
What makes this breakthrough more than just another entry in the annals of medical discovery is the paradigm shift it represents.
It challenges the long-held assumption that bacteria must be killed to be controlled. It redefines the enemy not as an organism, but as a behavior—a set of strategies bacteria use to outwit the immune system. And it underscores the importance of working with the body’s natural defenses rather than overriding them.
In this light, the discovery of NM102 is not just about molecules, proteins, or patents. It’s about reimagining our relationship with the microbial world—recognizing that, while some bacteria are enemies, most are allies, and that surgical strikes will always be better than carpet bombing.
The War Isn’t Over, But the Strategy Has Changed
As the global health community scrambles to contain the growing threat of antibiotic resistance, discoveries like NM102 are rays of hope. They remind us that innovation doesn’t always mean stronger weapons—it can also mean smarter ones.
The INRAE-led consortium has not only opened a new front in the war against superbugs; they’ve offered us a powerful new strategy—one that disarms instead of destroys, protects instead of poisons, and ensures that we preserve the microbial allies we depend on.
The future of antibiotics may not lie in obliteration, but in clever sabotage. And if NM102 is any indication, that future has already begun.
Reference: Seav-Ly Tran et al, An anti-virulence drug targeting the evolvability protein Mfd protects against infections with antimicrobial resistant ESKAPE pathogens, Nature Communications (2025). DOI: 10.1038/s41467-025-58282-8
Loved this? Help us spread the word and support independent science! Share now.