For the first time in scientific history, researchers have captured the exact instant DNA begins to unravel—an atomic-scale event that marks the genesis of life’s most fundamental process: replication. This breakthrough, spearheaded by a team at King Abdullah University of Science and Technology (KAUST), offers an unprecedented view of the molecular choreography that allows cells to copy their genetic blueprint with astonishing fidelity.
Published in Nature, the study peels back the curtain on how life perpetuates itself at the most granular level. Imagine witnessing the starting pistol of the race that sustains life—this is what these scientists have accomplished. They recorded, with atomic precision, the mechanical and chemical ballet of DNA unwinding, a critical first step in DNA replication. Without it, life as we know it would come to a standstill.
DNA: The Blueprint of Life Needs an Opening Act
To appreciate this discovery, we need to revisit DNA’s iconic structure. When James Watson and Francis Crick unveiled the double helix in 1953, they ignited a molecular revolution. Their model revealed DNA as two intertwined strands, encoding life’s instructions. But this elegant architecture posed a problem: for DNA to copy itself and pass those instructions to the next generation of cells, the strands must first separate—like opening a zipper before duplicating its teeth.
Enter helicase, the molecular machine charged with unzipping DNA. Scientists have known for decades that helicases are vital for unwinding the helix, but exactly how these tiny engines perform this feat has remained a mystery. That is, until now.
Peering Into the Nanoscopic World: The Role of Cryo-EM and Deep Learning
Using state-of-the-art cryo-electron microscopy (cryo-EM), paired with deep learning algorithms, KAUST researchers have captured helicase in action. Think of cryo-EM as a molecular camera that freezes biological molecules mid-motion, allowing scientists to observe their shapes and shifts in exquisite detail. Then, deep learning—artificial intelligence modeled on human brains—sifts through these snapshots to piece together the molecular story.
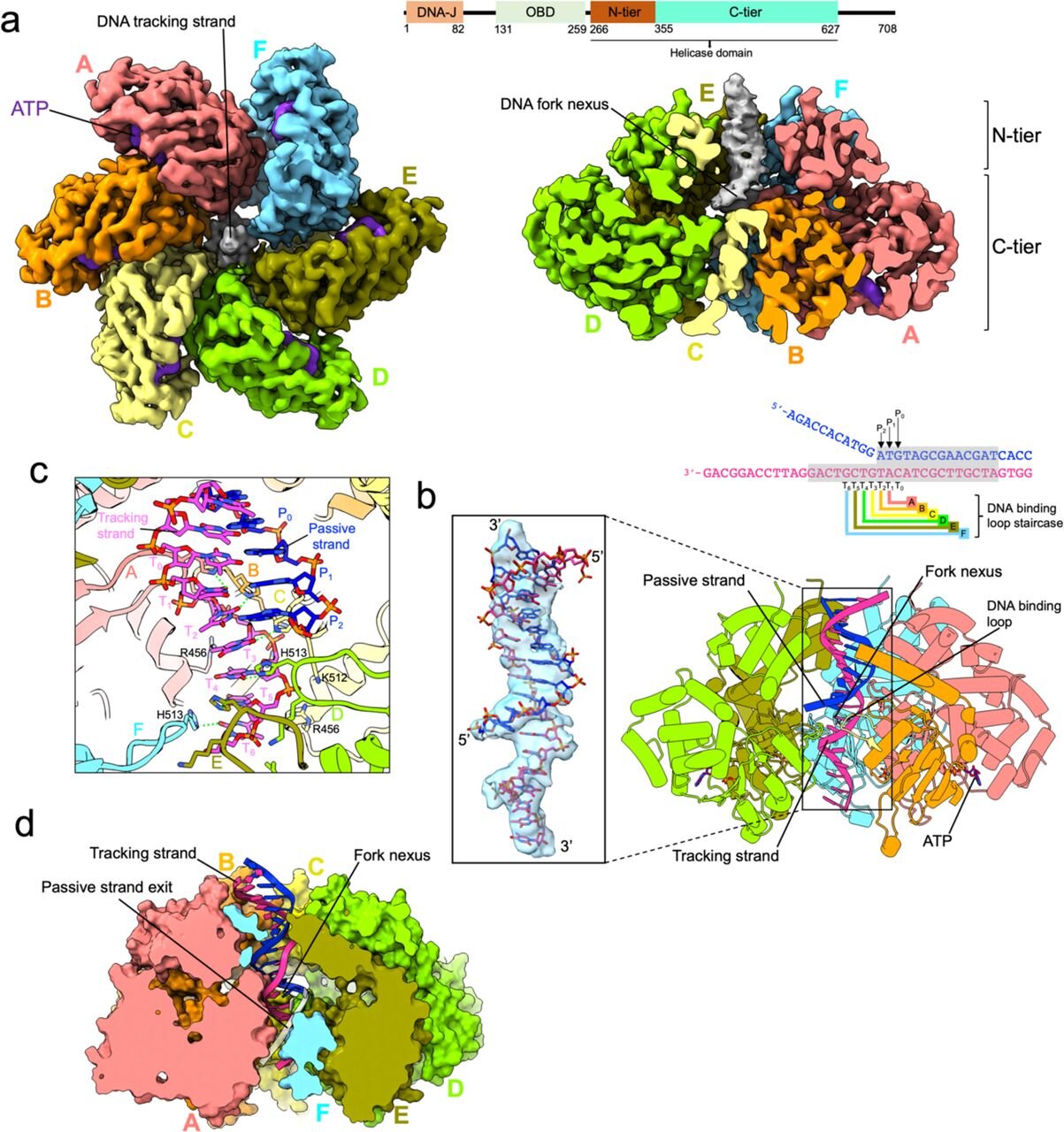
At the center of the KAUST study is the Simian Virus 40 Large Tumor Antigen (SV40 LTag), a well-known helicase. While this virus hijacks cellular machinery to replicate itself, its helicase offers a perfect model for studying DNA replication more broadly. What the KAUST scientists discovered is a series of 15 distinct atomic snapshots of helicase working its magic. For the first time, researchers can describe step-by-step exactly how DNA is unwound—how life’s most fundamental process begins.
Helicase: The Nanomachine That Powers Life
Helicase is no ordinary molecule. It’s a nanoscale machine, made of six protein subunits forming a ring that encircles DNA. Each subunit is powered by ATP (adenosine triphosphate), the energy currency of life. Just as gasoline powers the pistons of an engine, ATP drives helicase to perform its work.
But here’s the twist: helicase doesn’t wrench DNA apart in one dramatic move. Instead, it cycles through a series of precise conformational changes—shifts in shape powered by ATP hydrolysis (breaking down ATP to release energy). Imagine winding a spring-loaded toy: with each turn, tension builds until the spring releases energy in a burst of motion. In helicase, ATP fuels these mechanical transitions, destabilizing the bonds between DNA strands and pulling them apart.
“Helicase uses ATP not to pry DNA apart in one motion, but to cycle through conformational changes that progressively destabilize and separate the strands,” explains Dr. Alfredo De Biasio, assistant professor at KAUST and lead author of the study. “ATP burning, or hydrolysis, functions like the spring in a mousetrap, snapping the helicase forward and pulling the DNA strands apart.”
Two Helicases, Two Directions: The Perfect Molecular Coordination
One of the study’s most remarkable findings is that two helicases work in concert, binding DNA at separate sites to initiate the unwinding process from both directions. Since helicase can only move along DNA in one direction, this dual engagement allows replication to proceed bidirectionally. It’s like having two zippers on the same jacket, each moving in opposite directions but perfectly synchronized.
This coordination maximizes efficiency. Nature’s design ensures that the cell spends as little energy as possible while maintaining the accuracy necessary for life. The dual helicase mechanism also prevents potential bottlenecks in replication, reducing errors that could lead to mutations or cellular dysfunction.
ATP: The Fuel That Turns the Gears of Life
At the heart of this nanomachine is ATP. As helicase burns ATP, it removes physical constraints on its movement. Picture a lock that needs a key to release its mechanism; ATP acts as that key, freeing helicase to proceed along DNA’s length. As ATP is consumed, helicase increases the entropy—the disorder—of the system. This is the molecular equivalent of breaking an egg to make an omelet: creating disorder to allow for new order.
This ordered chaos is what lets helicase continue unwinding DNA, making way for other molecular players to enter the stage: DNA polymerases, which synthesize new strands; ligases, which stitch DNA fragments together; and proofreaders, ensuring the copy is faithful to the original.
More Than Biology: Lessons for Nanotechnology
While this research illuminates a fundamental process of life, it also offers inspiration for the future of technology. The helicase is not only a marvel of biology but also a model for engineered nanomachines.
“From a design perspective, helicases exemplify energy-efficient mechanical systems,” De Biasio notes. “Engineered nanomachines using entropy switches could harness similar energy-efficient mechanisms to perform complex, force-driven tasks.”
Imagine nanoscale machines, inspired by helicase, that could repair human cells, deliver medicine with pinpoint accuracy, or assemble tiny structures molecule by molecule. The potential is staggering, and nature provides the blueprint.
The Bigger Picture: Understanding Life’s Molecular Machinery
What makes this discovery so monumental is its dual impact: it answers fundamental questions about life while opening the door to futuristic technologies. By witnessing the very moment DNA unwinds, scientists gain critical insights into replication, mutation, and disease. This knowledge could lead to more effective cancer treatments—many cancers involve malfunctioning helicases—or new antiviral therapies.
Moreover, this atomic-level view offers a reminder of how intricate and well-tuned the machinery of life is. Every time a cell divides, this process occurs trillions of times over in living organisms, flawlessly orchestrated by nature’s molecular machines.
A Leap Forward in Science, A Step Toward the Future
Einstein once said, “The important thing is not to stop questioning.” This discovery is a testament to the spirit of scientific inquiry. The researchers at KAUST didn’t settle for what was already known about helicases; they wanted to see it happen, to understand it in motion.
Thanks to their work, we now have front-row seats to the molecular opening act of life. Helicase, once a shadowy figure in biology’s playbook, has stepped into the spotlight—revealing how life begins, one molecular twist at a time.
And perhaps, someday soon, humanity will build machines just as elegant, efficient, and life-changing.
Reference: Taha Shahid et al, Structural dynamics of DNA unwinding by a replicative helicase, Nature (2025). DOI: 10.1038/s41586-025-08766-w. www.nature.com/articles/s41586-025-08766-w