For most people, infections seem like short-lived battles—pathogens invade, the immune system responds, and either the infection is cleared or medical intervention helps control it. But in reality, the human body harbors a multitude of microbes that take a different approach. Instead of causing immediate harm, they slip into a latent stage, quietly embedding themselves in tissues, including the nervous system, where they can persist for years without triggering overt disease.
This long-term coexistence between host and pathogen remains one of the least understood aspects of infectious disease biology. Many scientists have assumed that once a pathogen reaches this dormant state, the immune system loses its ability to recognize or target it. However, a groundbreaking study led by researchers at the University of Pennsylvania School of Veterinary Medicine challenges this assumption, demonstrating that the immune system does, in fact, detect and respond to latent infections.
The study, published in Nature Microbiology, focuses on the parasite Toxoplasma gondii, the causative agent of toxoplasmosis. The findings provide new insights into how the immune system interacts with infections in the brain, opening the door to potential therapies for not only toxoplasmosis but also other persistent infections.
Toxoplasma Gondii: A Master of Stealth and Survival
Toxoplasma gondii is one of the most successful parasites on the planet, capable of infecting virtually all warm-blooded animals. However, felines are its definitive host, meaning that only in cats can the parasite undergo sexual reproduction. Most humans acquire T. gondii through the consumption of undercooked, contaminated meat or exposure to infected cat feces.
For healthy individuals, infection is often asymptomatic or results in mild flu-like symptoms. But for those who are immunocompromised—such as people with HIV/AIDS, organ transplant recipients, or individuals undergoing chemotherapy—T. gondii can cause severe neurological damage, leading to encephalitis and even death. The parasite also poses significant risks to pregnant women, as it can cross the placenta and harm the developing fetus.
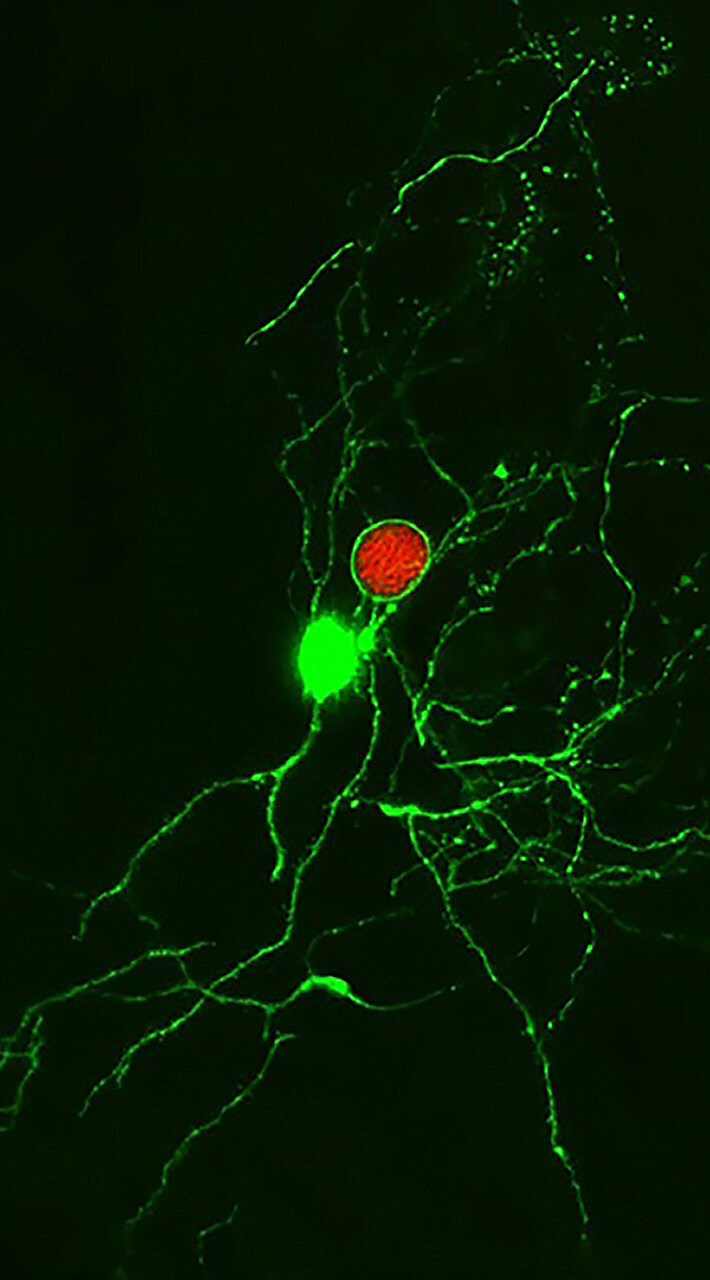
Once inside the body, T. gondii undergoes a transformation. It initially exists in a fast-replicating form known as the tachyzoite, which spreads quickly and infects various tissues. But as the immune system mounts a response, the parasite shifts into its latent stage, forming microscopic cysts within neurons. These cysts allow the parasite to evade immune detection and establish a long-term presence in the brain, where it can persist for a lifetime.
For decades, scientists believed that T. gondii cysts were completely shielded from immune attack. The prevailing theory suggested that neurons provided a sanctuary for the parasite, offering protection from immune surveillance. But the new research from Penn Vet turns this idea on its head.
The Immune System’s Unexpected Recognition of Latent Toxoplasma
Led by Christopher A. Hunter, a professor at Penn Vet, the research team discovered that the immune system does, in fact, recognize T. gondii cysts in the brain. Contrary to previous assumptions, certain T cells are capable of detecting and responding to these infected neurons.
The study found that when these T cells identified cyst-containing neurons, they triggered an immune response that helped control the parasite. However, the relationship between host and pathogen is a delicate one. The researchers observed that when cyst formation was disrupted—meaning the parasite remained in its active, replicating form instead—there was an even greater parasite burden and significantly more damage to brain tissue.
This suggests that T. gondii cysts may actually play a dual role: they help the parasite survive, but they also prevent it from overwhelming the host. In other words, cyst formation may be an evolutionary compromise, allowing the parasite to persist without killing its host. If the host were to die, the parasite’s own survival would be at risk.
Rethinking How the Brain Handles Persistent Infections
One of the most surprising aspects of the study was the discovery that neurons are not the impenetrable refuge for pathogens that scientists once thought. Julia N. Eberhard, a co-author and immunology doctoral student, explained that previous literature suggested T. gondii cysts could completely avoid immune recognition. However, the study’s findings challenge that idea, showing that the immune system can, in fact, detect and interact with infected neurons.
Another striking discovery involved a strain of T. gondii that was unable to transition into its cystic form. The expectation was that without cyst formation, the immune system would be able to clear the parasite more efficiently. Instead, the researchers found that the parasite persisted in mice for at least six months, proving that cyst formation is not strictly necessary for long-term infection. This unexpected result suggests that the immune system’s ability to clear T. gondii—whether in cyst form or not—is more complex than previously thought.
Using Mathematical Models to Decode Immune Interactions
To further understand these findings, the team turned to mathematical modeling, led by Aaron Winn, a doctoral student in the Department of Physics and Astronomy. The models helped explain how immune pressure on the latent stage of T. gondii influences the rise and fall of cyst numbers over time. These computational approaches reinforced the experimental observations, providing additional support for the idea that the immune system plays a more active role in managing latent infections than previously assumed.
Implications for Future Therapies and Broader Infectious Disease Research
The discovery that the immune system can recognize and target T. gondii cysts opens up exciting possibilities for future therapies. If scientists can develop treatments that enhance the body’s ability to clear latent cysts without causing excessive brain inflammation, it could lead to new strategies for treating toxoplasmosis.
Additionally, the findings have broader implications beyond T. gondii. Many persistent infections—including those caused by herpesviruses, cytomegalovirus, and even certain bacteria—enter latent stages within the nervous system. Since some of these infections do not have viable animal models, T. gondii provides a valuable system for studying how the immune system interacts with latent pathogens in the brain.
Lindsey A. Shallberg, a co-author of the study, emphasized that T. gondii serves as a tractable model that can be studied in the lab. By understanding how the immune system interacts with latent parasites in this context, scientists may be able to apply these insights to other infections that evade immune detection in a similar manner.
What Comes Next? The Ongoing Quest to Understand Brain Immunity
Moving forward, Hunter and his team plan to investigate whether T cells directly recognize infected neurons and to explore the mechanisms by which the immune system controls latent T. gondii infections. By unraveling these complex interactions, they hope to shed light on how the nervous system responds to persistent infections and whether immune-based therapies could one day help clear them.
The study’s findings challenge long-standing assumptions about the immune system’s role in latent infections, reshaping our understanding of how pathogens survive in the brain. With further research, scientists may uncover new ways to combat chronic infections, bringing us closer to effective treatments for toxoplasmosis and other persistent diseases.
This revelation serves as a reminder that even in its quietest moments, the immune system remains engaged in a delicate and ongoing battle, working to keep hidden invaders at bay.
Reference: Julia N. Eberhard et al, Immune targeting and host-protective effects of the latent stage of Toxoplasma gondii, Nature Microbiology (2025). DOI: 10.1038/s41564-025-01967-z