As we age, it’s not just our skin that wrinkles or our joints that become stiffer. Our bones, too, undergo significant changes—changes that are often subtle but impactful, causing our bodies to feel more creaky and fragile over time. One of the most significant aspects of this age-related transformation lies within the very cells that keep our bones strong and functional: osteocytes. Recent groundbreaking research has uncovered fascinating details about how these cells change as we get older, offering new insights into how age-related bone loss, including conditions like osteoporosis, occurs.
In a recent study conducted by researchers from the University of Texas at Austin, in collaboration with the Mayo Clinic and Cedars-Sinai Medical Center, a new breakthrough has been made in understanding bone aging. This study, published in Small and Aging Cell, shines a light on how osteocytes—known as the master regulators of bone health—undergo dramatic changes as they age, impairing their ability to maintain healthy bone structure. Their findings not only reveal what happens to osteocytes with age but also offer exciting new possibilities for future treatments for bone conditions such as osteoporosis.
Osteocytes: The Master Regulators of Bone Health
To understand the significance of this study, it’s essential to grasp the role of osteocytes in our bones. These cells, embedded within the bone matrix itself, act as the command center for maintaining bone strength. Osteocytes sense mechanical forces on the bone, such as pressure and stress, and direct other bone cells—osteoblasts (which build bone) and osteoclasts (which break down bone)—on when to build up or break down bone tissue. This process of bone remodeling is crucial for maintaining bone density and integrity.
However, with age and exposure to stressors, osteocytes themselves begin to change. In particular, the study highlighted a crucial phenomenon: the onset of cellular senescence in osteocytes. Cellular senescence refers to a state where cells stop dividing and proliferating, yet they don’t die off. These senescent cells accumulate over time and can disrupt normal cellular function. This phenomenon is not limited to osteocytes but affects many cell types in the body and is believed to play a significant role in aging and age-related diseases.
The Impact of Aging on Osteocytes
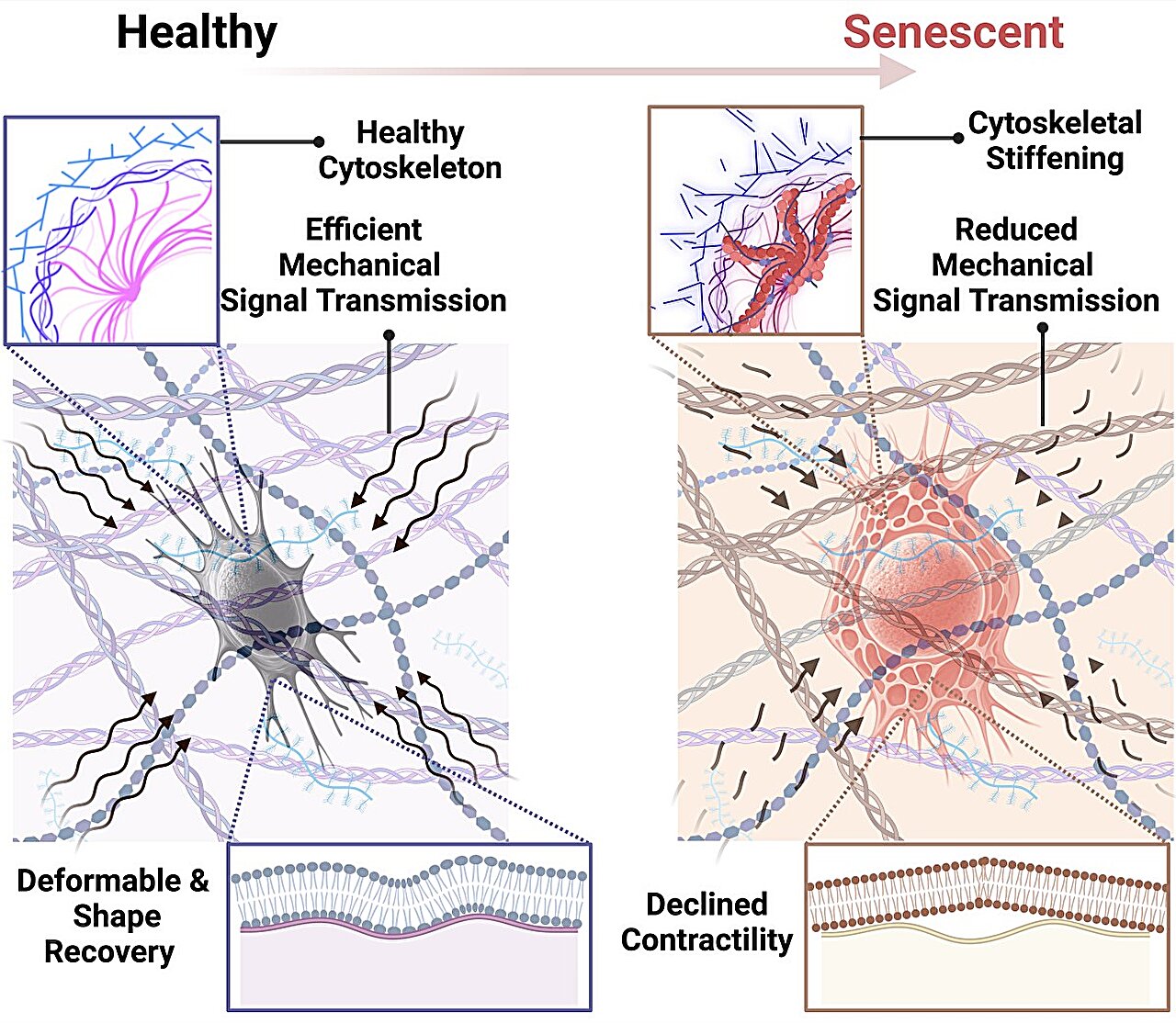
As osteocytes age, they become increasingly susceptible to cellular senescence. The team led by Maryam Tilton, an assistant professor in the Cockrell School of Engineering at the University of Texas, uncovered the profound impact this has on the structural and functional capabilities of osteocytes. These aging osteocytes undergo a stiffening of their internal structure, specifically within their cytoskeleton—the network of protein filaments that maintain the cell’s shape and enable its movement and interaction with the environment.
Think of the cytoskeleton as the internal scaffolding of a building. Just as a building’s structure must remain flexible to adapt to changing conditions and stresses, osteocytes need a flexible cytoskeleton to respond appropriately to mechanical forces. When the cytoskeleton of osteocytes stiffens with age, it loses the ability to effectively detect and respond to changes in mechanical stress on the bones. This disrupts the delicate balance of bone remodeling, leading to a weakening of the bone structure, contributing to bone fragility.
“The scaffolding inside the cell, when it becomes rigid, makes it harder for the osteocyte to adapt and function as it should,” said Tilton. “Just as a building with a rigid structure can’t cope with shifting stresses, stiffened osteocytes can’t regulate bone remodeling effectively, which leads to bone loss.”
This stiffening of osteocytes isn’t just a structural problem; it’s also a functional one. The aging osteocytes become less responsive to mechanical cues, like the pressure of walking or the force of lifting, which are normally signals to either strengthen or break down bone tissue. When these cells no longer function as they should, the bones become more vulnerable to fractures and conditions like osteoporosis.
The Role of Senescent Cells and Inflammation
Aging osteocytes also have to contend with the presence of other senescent cells in the body. Senescent cells are known for their toxic secretion of molecules collectively referred to as the senescence-associated secretory phenotype (SASP). These molecules trigger inflammation and damage in surrounding tissues, contributing to the degenerative processes seen in various age-related conditions, including cancer, cardiovascular disease, and, of course, osteoporosis.
In the case of osteocytes, the accumulation of senescent cells leads to a cascade of detrimental effects. The SASP molecules released by these cells create an inflammatory environment that further exacerbates the stiffening of osteocytes, weakening bone health. The more senescent cells accumulate, the more osteocytes lose their ability to regulate bone remodeling and maintain bone strength.
New Approaches to Treating Osteoporosis and Age-Related Bone Loss
The exciting aspect of this new research is the potential it holds for improving treatments for osteoporosis and other bone-related conditions. Osteoporosis, which affects millions of people worldwide—particularly postmenopausal women and the elderly—is characterized by weakened bones that are prone to fractures. As our global population ages, osteoporosis and age-related bone loss are becoming increasingly prevalent, making it more crucial than ever to understand the mechanisms behind these conditions.
Most current treatments for osteoporosis focus on slowing down the breakdown of bone or stimulating bone formation, but these therapies don’t address the root cause—the changes happening within the osteocytes themselves. This new research opens the door to novel approaches that target the aging osteocytes directly.
Tilton and her team suggest that by understanding the mechanical properties of osteocytes, researchers can explore ways to reverse the damage caused by cellular senescence. Much like physical therapy can help restore movement in stiffened joints, the researchers propose that mechanical cues could be used to help osteocytes regain their flexibility and ability to sense and respond to mechanical forces. This could potentially reverse the negative effects of aging on osteocytes, leading to stronger, healthier bones.
In addition to investigating mechanical approaches, the research team is also exploring the possibility of using biomechanical markers to identify senescent osteocytes and develop therapies to either clear them from the body or restore their function. This approach offers an alternative to current drug-based treatments, such as senolytic therapies, which aim to target and remove senescent cells but can be limited in effectiveness.
A Future of Better Bone Health
The potential implications of this research extend far beyond simply improving osteoporosis treatments. As we learn more about how bones age and how osteocytes change over time, new avenues open up for treating a range of age-related conditions, not just those affecting bones but also those tied to inflammation and cellular damage. If osteocytes could be rejuvenated or selectively cleared of senescent cells, it might not only improve bone health but also help slow down the broader aging process and mitigate age-related diseases.
Looking ahead, the team plans to expand their research by exploring how various stressors—such as mechanical, environmental, and hormonal factors—affect osteocytes and whether these stressors can be controlled or mitigated to protect bone health. They also aim to test different therapeutic interventions that could restore osteocyte function, offering hope for more effective treatments for osteoporosis, fractures, and other conditions related to bone aging.
As we continue to uncover the mysteries of aging, this study offers an exciting glimpse into the future of bone health and aging. By focusing on the cellular mechanics of osteocytes and how they change over time, researchers may soon be able to offer more targeted, personalized treatments for osteoporosis and other age-related diseases, leading to better health outcomes for millions of people as they grow older.
References: Maryam Tilton et al, Tracing Cellular Senescence in Bone: Time‐Dependent Changes in Osteocyte Cytoskeleton Mechanics and Morphology, Small (2025). DOI: 10.1002/smll.202408517
Maryam Tilton et al, Stiffening symphony of aging: Biophysical changes in senescent osteocytes, Aging Cell (2024). DOI: 10.1111/acel.14421