For decades, senescent skin cells—often nicknamed “zombie cells”—have haunted the imaginations of scientists trying to decode the mysteries of aging. These cells, which have stopped dividing but stubbornly refuse to die, embody a paradox: they simultaneously harm and heal. They are the silent saboteurs behind chronic inflammation and degenerative diseases, yet also the indispensable guardians that help repair wounds and maintain tissue health.
Now, groundbreaking research from Johns Hopkins University offers an exciting new twist in the tale: not all zombie cells are created equal. In a study published in Science Advances, scientists report the discovery of three distinct subtypes of senescent skin cells—each with its own unique fingerprint of form, function, and fate. This discovery could mark a critical turning point, empowering researchers to develop smarter, more selective therapies that eliminate the villains among these cells while preserving their helpful counterparts.
A Deeper Dive into the Zombie State
Traditionally, scientists have viewed senescence as a binary switch. A skin cell was either youthful and healthy or senescent and dysfunctional—no shades of gray in between. Yet that black-and-white perspective failed to capture the true complexity of what happens when cells age.
“We’ve known that senescent skin cells are different from senescent immune or muscle cells,” explains Jude Phillip, assistant professor of biomedical engineering at Johns Hopkins. “But what’s surprising is that even within skin cells, senescence can take multiple forms. One skin cell might become a very different kind of zombie than another.”
This new insight turns the entire understanding of cellular aging on its head. Senescence, it appears, is not a single path but a crossroads where cells might wander down several diverging trails, each leading to a different kind of aged existence.
Peering Beneath the Surface with Cutting-Edge Technology
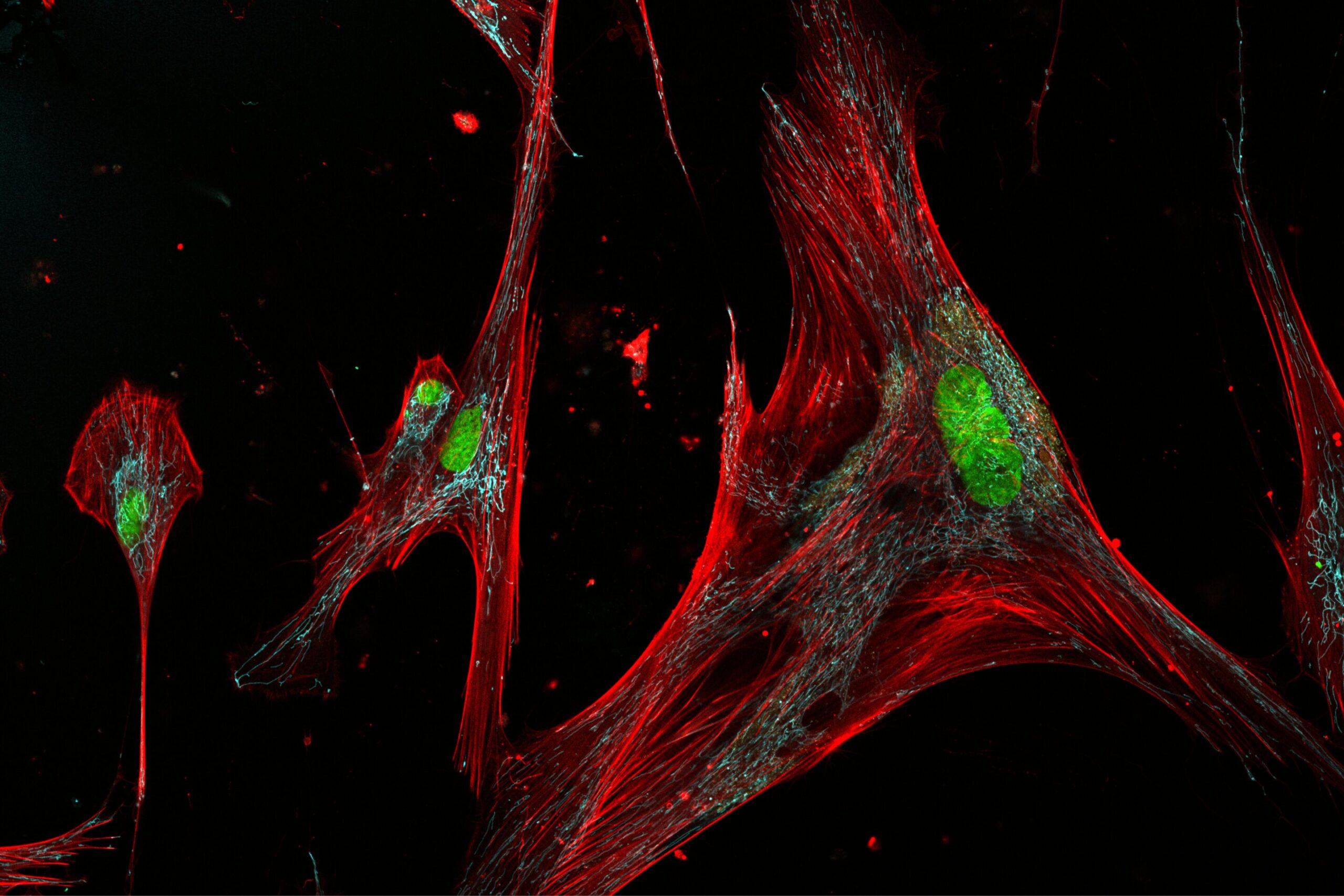
Uncovering the hidden diversity of zombie cells was no simple task. The team leveraged powerful new tools at the intersection of machine learning, advanced imaging, and biomedical engineering.
The researchers gathered skin cell samples—specifically fibroblasts, the builders of the skin’s scaffolding—from 50 healthy donors aged 20 to 90 years, all participants in the Baltimore Longitudinal Study of Aging, the longest-running study of human aging in the United States.
In the lab, they exposed these fibroblasts to DNA damage, simulating the wear and tear that naturally accumulates over a lifetime. As expected, many cells slipped into senescence. But instead of simply labeling them as “senescent” and moving on, the researchers took a much closer look.
By applying specialized fluorescent dyes, they visualized telltale signs of senescence inside the cells. Then, with the help of newly developed algorithms, they measured a staggering 87 distinct physical characteristics per cell—from size and shape to internal molecular markers.
What emerged from the data was a revelation: fibroblasts adopted 11 different shapes and sizes as they aged, and among those, three were clearly distinct subtypes of senescent cells. Intriguingly, only one subtype, dubbed C10, showed a strong connection to older age.
The Good, the Bad, and the Potentially Ugly
Why does this matter? Because each of these zombie cell subtypes behaves differently—and that could be the key to unlocking safer and more effective anti-aging therapies.
When the researchers treated the senescent cells with Dasatinib and Quercetin—a drug combination currently being tested in clinical trials to eliminate senescent cells—they observed something striking. The therapy was very effective against one subtype (C7) but much less successful at eliminating the age-associated C10 subtype.
This finding is profound: it suggests that some zombie cells are more vulnerable to certain drugs than others. In the future, scientists might be able to design precision senotherapies that target only the harmful senescent cells—those that fuel inflammation and disease—while sparing or even nurturing the beneficial ones that help with tissue repair.
“We now have a toolkit to start developing more targeted treatments,” says Phillip. “Instead of using a sledgehammer to eliminate all senescent cells, we can use a scalpel.”
Aging, Cancer, and the Promise of Smarter Treatments
The implications extend far beyond skincare or simple anti-aging remedies. Cancer treatments, in particular, could be transformed by this new understanding.
Some cutting-edge therapies aim to push cancer cells into senescence—trapping them in a non-dividing, zombie-like state to prevent tumor growth. However, these therapies also risk leaving behind hordes of senescent cells, which can secrete inflammatory molecules that worsen the patient’s overall health.
Likewise, traditional chemotherapy unintentionally pushes healthy cells, like fibroblasts, into senescence as a side effect. These senescent cells can linger, undermining recovery by promoting tissue damage and chronic inflammation at a time when the immune system is already weakened.
Imagine a future where, after a round of chemotherapy, a patient could be given a precision senotherapy that sweeps away only the dangerous zombie cells—leaving behind those that are still contributing to healing. The potential to reduce side effects, accelerate recovery, and improve long-term outcomes could be enormous.
The Next Frontier: From Petri Dish to Patient
While these results are thrilling, the researchers caution that much work remains. So far, their analysis has focused on fibroblasts grown in laboratory dishes. The next step is to confirm that these three zombie cell subtypes exist—and behave similarly—in actual human tissues.
“Cells can act differently when they’re in the body compared to a petri dish,” Phillip explains. “We want to study tissue samples directly, especially from skin affected by different age-related diseases.”
If successful, the team hopes to use this knowledge to develop predictive diagnostics—tools that could one day tell doctors which senescent cell subtypes are present in a patient’s tissues and recommend the most effective, personalized treatments.
Zombie Cells: From Villains to Complex Characters
This study marks a fundamental shift in how we think about senescence. No longer are zombie cells merely unwanted relics of aging. They are complex, nuanced players—some harmful, some helpful, and all part of the intricate symphony of life and decay.
As scientists continue to unravel their secrets, we may inch closer to not just extending lifespan, but enhancing healthspan—the years we live free from the burdens of disease, frailty, and decline.
The dream is no longer science fiction. It’s science—advancing, step by thrilling step, toward a future where the once-inevitable march of aging could be slowed, softened, or even partially reversed.
“We’re moving toward a world where we don’t just treat symptoms of aging,” Phillip says. “We treat its root causes.”
In the battle against time, the real enemy might not be aging itself—but failing to understand the hidden complexity within our own living cells. Thanks to this research, humanity has taken a vital stride closer to mastering that complexity—and perhaps, one day, conquering it.
Reference: Pratik Kamat et al, Single-cell morphology encodes functional subtypes of senescence in aging human dermal fibroblasts., Science Advances (2025). DOI: 10.1126/sciadv.ads1875. www.science.org/doi/10.1126/sciadv.ads1875
Loved this? Help us spread the word and support independent science! Share now.