In the quiet corners of university labs, where curiosity meets tenacity, science sometimes stumbles across nature’s best-kept secrets. One such revelation has emerged from the University of Oregon, where researchers have discovered that a common yeast living on human skin produces a powerful antimicrobial compound—one that could become a key ally in the global battle against antibiotic-resistant infections.
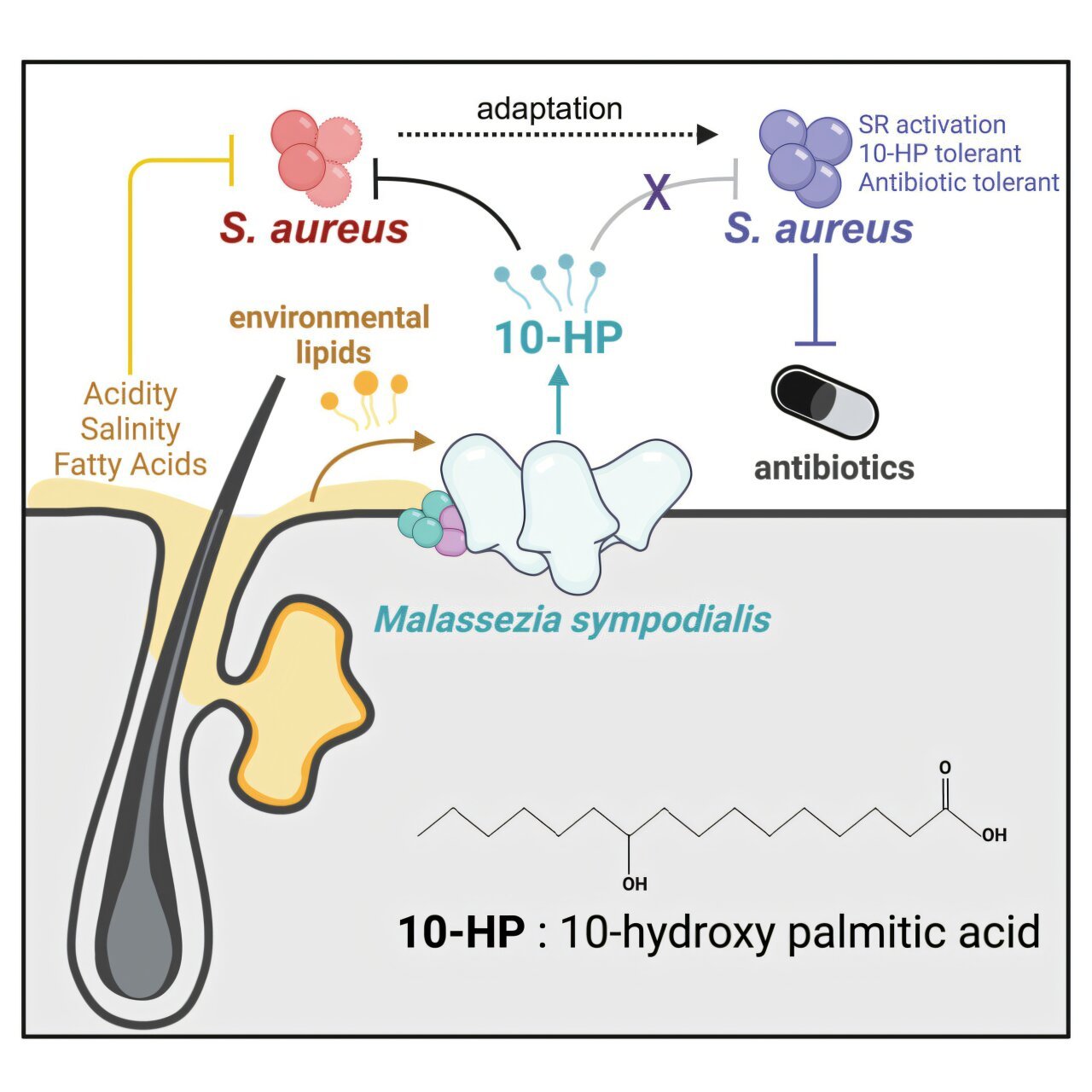
At the heart of this breakthrough is a seemingly unremarkable skin fungus: Malassezia. It’s been quietly living on our skin for millennia, feeding on the natural oils and fats that coat our epidermis. But as postdoctoral researcher Dr. Caitlin Kowalski has now shown, this humble microbe may be wielding biochemical weapons against dangerous bacteria—especially the formidable Staphylococcus aureus, a pathogen responsible for nearly half a million hospitalizations in the U.S. each year.
A War on the Skin’s Surface
Human skin, far from being a barren barrier, is a battlefield. It hosts a vibrant, competitive ecosystem of microorganisms—a complex microbiome made up of bacteria, fungi, and viruses—all of them jostling for space and resources. Among these organisms, Malassezia species dominate the fungal landscape. Though often associated with conditions like dandruff or eczema, they are, for the most part, benign cohabitants. They have evolved in lockstep with us, perfectly adapted to life on skin rich in lipids, where they scavenge oils they cannot synthesize on their own.
In a study recently published in Current Biology, Kowalski and her team revealed that these fungi do far more than live passively on the surface. They are biochemical powerhouses, transforming skin lipids into antimicrobial agents that can decimate certain bacterial invaders—most notably Staphylococcus aureus, also known as staph.
“It’s a very different approach to antibiotic discovery,” said Kowalski. “Instead of looking out into the wild for exotic microbes in soil or deep-sea vents, we’re turning inward—toward the communities already living on us.”
The Fungal Assassin
The specific strain under the microscope was Malassezia sympodialis, a yeast that thrives on healthy human skin. When Kowalski exposed this fungus to lipid-rich environments that mimic human skin, it began producing a group of compounds known as hydroxy fatty acids. These molecules, while chemically simple, had a potent effect: they dismantled the cell membranes of S. aureus, essentially tearing them apart and causing the bacterial contents to leak out—a cellular death sentence.
What makes this finding especially exciting is the selective toxicity. While many antibiotics act broadly and wipe out both harmful and beneficial bacteria, the hydroxy fatty acids showed specificity against S. aureus. This precision could mean fewer side effects and a reduced likelihood of disrupting the broader microbiome.
“These hydroxy fatty acids act like detergents,” Kowalski explained. “They literally dissolve the bacteria’s membrane. And they do it in minutes.”
This rapid action could make them ideal candidates for topical applications in clinical settings—particularly where antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA), threaten immunocompromised patients.
The Power of pH
One surprising twist in the study was the realization that acidity matters—a lot. In typical lab conditions, many antimicrobial candidates fall flat. But when Kowalski and her colleagues adjusted the pH to match the acidic environment of healthy human skin, they saw a dramatic uptick in activity. What appeared benign in a neutral petri dish suddenly became lethal under more realistic conditions.
“This might be why similar antimicrobial mechanisms have been missed before,” Kowalski noted. “We don’t always replicate the conditions of the human body closely enough in the lab.”
Human skin, with its naturally low pH, may be the perfect staging ground for these microbial battles—a hidden layer of defense we’ve only begun to appreciate.
The Superbug Fight Isn’t Over
Yet, as with all battles, there’s a catch. While the hydroxy fatty acids were deadly to S. aureus at first, prolonged exposure led to bacterial adaptation. After repeated encounters, the bacteria developed tolerance—a chilling reminder that even natural antibiotics can provoke resistance, just like synthetic ones.
Through genetic analysis, the team identified mutations in the Rel gene of the staph bacteria. This gene is known to activate stress responses in microbes, helping them survive in hostile environments. Strikingly, similar mutations have been observed in clinical cases of antibiotic-resistant S. aureus, underscoring the real-world relevance of the findings.
“This shows us that bacterial resistance isn’t just a laboratory curiosity—it can evolve from interactions with other microbes in the body,” Kowalski said. “We need to consider this when designing new treatments.”
Skin’s Secret Chemistry
Beyond the implications for antimicrobial discovery, the study sheds light on a largely ignored branch of microbiome research. While the gut microbiome has become a scientific darling over the past two decades—linked to everything from mood to metabolism—the skin microbiome has lagged behind in attention. Yet it is an equally dynamic system.
“Skin is a lipid-rich environment,” said Kowalski. “And the microbes living there aren’t just passive—they’re processing those lipids and turning them into new, bioactive compounds.”
By viewing the skin microbiome as an active biochemical system rather than a static population of microbes, researchers can start to unlock its therapeutic potential. The implications go far beyond staph infections. Similar mechanisms could be involved in regulating inflammation, controlling acne, or even preventing fungal infections.
A Team Effort in Discovery
The road to identifying the antimicrobial compounds was anything but straightforward. “It was like finding a needle in a haystack,” said Matthew Barber, Kowalski’s adviser and an associate professor of biology at the University of Oregon. “Except the needle was a molecule, and we had no idea what it looked like.”
The research drew on expertise from multiple disciplines—biochemistry, molecular biology, microbiology, and analytical chemistry. Kowalski collaborated with scientists at McMaster University, whose chemical microbiology tools helped isolate and analyze the elusive hydroxy fatty acids. The result was a detailed chemical fingerprint of the compounds involved and a better understanding of how Malassezia processes skin lipids into antimicrobials.
Fungi as Pharmacies
Fungi have long been a rich source of antibiotics—penicillin, after all, came from a mold. But human-associated fungi remain largely unexplored compared to their soil-dwelling cousins. This research is a call to arms to change that.
“What excites me is the idea that fungi on our bodies may be creating molecules with therapeutic potential,” said Kowalski. “And we’ve barely scratched the surface.”
Unlike synthetic compounds that must be tested for compatibility with human biology, these natural substances are already part of our skin’s chemical milieu. That makes them promising candidates for safe, effective medicines.
Looking Ahead: A New Frontier for Antibiotics
As Kowalski prepares to launch her own lab, her focus will remain on the unsung heroes of the skin microbiome. She hopes to investigate how fungi interact with the immune system, how microbial communities shape skin diseases, and how we might harness the chemistry of commensal organisms to prevent or treat infections.
Barber, her mentor, emphasized the urgency of this work: “Antibiotic-resistant infections are among the biggest threats to human health. We need more options. And we need to look everywhere—including right on our skin.”
In a world increasingly vulnerable to superbugs, this discovery is more than a scientific curiosity. It’s a glimpse into a new paradigm—one where our own microbial companions could help us survive the coming storm of drug-resistant diseases.
Conclusion: Nature’s Hidden Arsenal
The discovery that Malassezia fungi can produce compounds lethal to dangerous bacteria reminds us that some of the best solutions may be hiding in plain sight. As we face an antibiotic crisis that threatens to roll back a century of medical progress, innovations like this shine a light on the untapped potential of the human microbiome.
Our skin is more than just a shield; it’s a laboratory, a battleground, and perhaps even a pharmacy. If we listen closely to the microbes that call us home, they may just teach us how to heal.
Reference: Caitlin H. Kowalski et al, Skin mycobiota-mediated antagonism against Staphylococcus aureus through a modified fatty acid, Current Biology (2025). DOI: 10.1016/j.cub.2025.03.055
Loved this? Help us spread the word and support independent science! Share now.