The mammalian immune system is often lauded for its extraordinary ability to recognize and combat harmful invaders like viruses, bacteria, and even cancer cells. However, there are more subtle, yet equally crucial, roles the immune system plays, particularly in maintaining the delicate balance of metabolic processes. One fascinating and lesser-known function involves a special subset of immune cells known as regulatory T cells (Tregs), which help to maintain homeostasis in fat tissue. This area of research, while still in its infancy, has the potential to lead to groundbreaking new strategies for treating obesity—a major global health crisis affecting over a billion people worldwide.
In a recent study published in Science Immunology, researchers from Emory University in Atlanta have uncovered critical insights into how Tregs play a dynamic role in regulating fat tissue homeostasis, particularly in visceral adipose tissue (VAT)—a type of fat found around internal organs such as the liver, stomach, and intestines. This research could open doors to new treatments for obesity and its associated diseases, including cardiovascular disease, type 2 diabetes, and certain cancers.
The Immune System and Fat Cells: A Surprising Connection
At the core of this study is the recognition that immune cells, particularly Tregs, are not just stationed in the bloodstream or lymph nodes waiting for invaders but also in specific tissues like VAT. These tissue-resident T cells play a vital role in the function and stability of the tissues in which they reside. In the case of VAT, Tregs are essential for maintaining metabolic balance, ensuring that fat cells function in harmony with the body’s broader metabolic needs. They help prevent overaccumulation of fat, support insulin sensitivity, and reduce chronic inflammation—critical factors that influence conditions like obesity and metabolic syndrome.
Visceral adipose tissue, which surrounds the internal organs, is often described as a “bad” form of fat because it is strongly linked to various health problems. Unlike subcutaneous fat, which sits just beneath the skin and is generally considered less harmful, VAT has a direct line to the bloodstream, influencing processes like hormone secretion and inflammatory responses. When VAT becomes excessive, it triggers a cascade of metabolic dysfunction, contributing to the onset of chronic diseases such as type 2 diabetes, hypertension, and atherosclerosis.
What makes Tregs so important in this context is their role in preventing VAT from tipping into a state of dysfunction. These cells maintain a healthy immune environment in fat tissue by keeping inflammation in check and ensuring that fat cells do not become excessively enlarged, which can cause them to secrete pro-inflammatory molecules. In short, Tregs help keep VAT in balance, supporting overall metabolic health.
The Mystery of Missing Tregs in Obesity
The Emory University team’s investigation began with a deceptively simple question: Why do Tregs disappear from VAT in people with obesity? This question is pivotal because the loss of Tregs from fat tissue can contribute to the systemic inflammation that characterizes obesity-related diseases. Previous studies had shown that Tregs are present in healthy VAT, but they are conspicuously absent or severely diminished in obese individuals. In some cases, fat samples from obese people showed no trace of Tregs at all.
To explore this mystery, the Emory researchers turned to animal models of obesity, tracking the behavior of Tregs in various metabolic states. Their findings were startling: Obesity, they discovered, has a direct impact on the cholesterol metabolism of Tregs, leading to an increase in inflammation and insulin resistance—two hallmark features of obesity-related diseases.
A crucial discovery was that obesity disrupts cholesterol homeostasis in Tregs. Cholesterol, which is typically thought of as merely a building block for cellular membranes or a precursor for hormones like estrogen and testosterone, plays a vital role in regulating immune cell function. Tregs, like many other immune cells, rely on a precise balance of cholesterol to maintain their stability and function. In the case of obesity, however, this balance is thrown off, leading to an inflammatory response in VAT that contributes to the dysfunction of fat cells.
Unveiling the Mechanism: A Master Regulator of Cholesterol
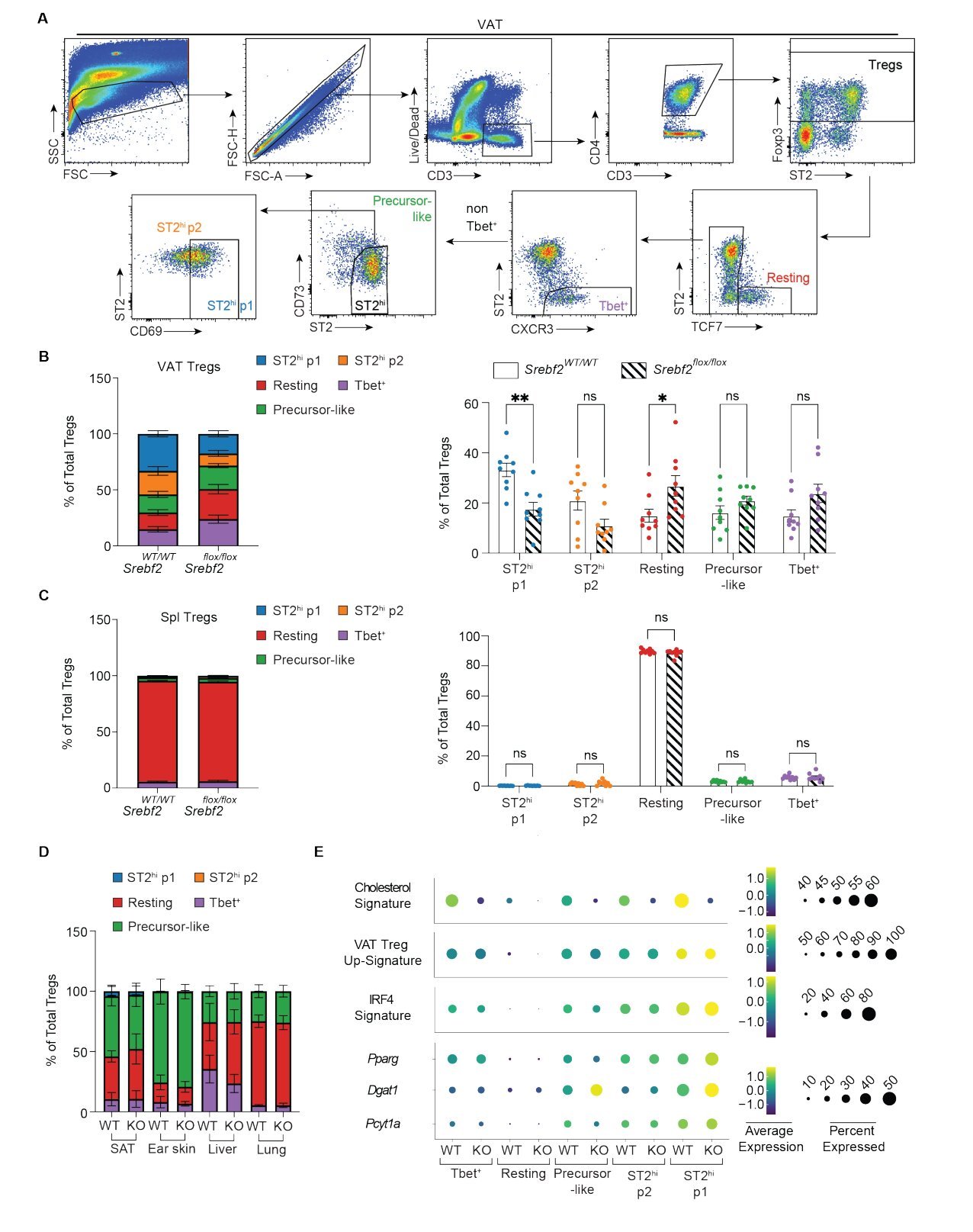
To understand how obesity affects Tregs, the researchers focused on the master regulator of cholesterol metabolism—SREBP-2 (Sterol Regulatory Element-Binding Protein 2). SREBP-2 is a transcription factor that controls the expression of genes involved in cholesterol synthesis and uptake. In obese animals, the Emory team found that the expression of SREBP-2 in VAT was impaired. This disruption of cholesterol homeostasis had a profound effect on the population of Tregs, specifically those known as St2hi VAT Tregs.
These St2hi VAT Tregs, named for their expression of the receptor St2, are critical to maintaining the balance and function of VAT. The Emory team discovered that when cholesterol metabolism in these Tregs was disrupted in obese mice, there was a marked decline in the number of these key immune cells within the fat tissue. The absence of Tregs in VAT contributed to the chronic inflammation and metabolic dysfunction that are central to obesity-related diseases.
Interestingly, the researchers also found that by restoring cholesterol homeostasis in Tregs, they could rescue the accumulation of Tregs in VAT. This finding is a game-changer, as it suggests that targeting cholesterol metabolism could be a potential therapeutic strategy for reversing the metabolic disturbances seen in obesity.
The Broader Implications: Targeting Cholesterol in Obesity Treatment
The implications of these findings are profound. If the loss of Tregs from VAT is indeed driven by disrupted cholesterol homeostasis, it opens up new possibilities for treating obesity and its associated diseases. One potential avenue is the modulation of cholesterol metabolism in Tregs, a strategy that could help restore Treg populations in VAT and reduce inflammation.
In fact, the research suggests that cholesterol-targeted therapies could be a promising approach for treating obesity-related metabolic diseases. By restoring the normal function of Tregs in fat tissue, it may be possible to mitigate the harmful effects of obesity, such as insulin resistance and systemic inflammation, without relying on more invasive treatments like weight loss surgery or harsh pharmaceutical interventions.
This research also highlights the intricate relationship between the immune system and metabolic processes. It underscores the importance of understanding not just the immune response to infections or cancers but also how immune cells interact with metabolic tissues like fat. Tregs, it seems, are not just defenders of the body against pathogens—they are also essential regulators of metabolic health, ensuring that fat cells function properly and that systemic inflammation is kept in check.
A Broader Context: The Role of Tissue-Resident T Cells
The study at Emory University is part of a broader body of research into the role of tissue-resident T cells in maintaining tissue homeostasis. These specialized immune cells do not circulate through the lymphatic system like other T cells. Instead, they reside in specific tissues where they perform critical functions related to tissue stability and immune regulation. In addition to their role in fat tissue, tissue-resident Tregs have been identified in various other organs, including the lungs, intestines, skin, and muscles.
For example, a 2023 study from Harvard Medical School demonstrated that tissue-resident Tregs in muscle tissue protect mitochondria and guard muscle cells from stress induced by exercise. This research further highlights the diverse roles that tissue-resident Tregs play in maintaining health across different organ systems. Whether in fat, muscle, or other tissues, these immune cells are vital to the proper functioning of our organs and to the maintenance of metabolic homeostasis.
Looking Ahead: Potential Therapies and Future Research
The discoveries made by the Emory team open up exciting new avenues for obesity treatment. As they continue to explore the relationship between Tregs, cholesterol metabolism, and obesity, it is likely that new therapies will emerge that can restore immune balance in fat tissue and mitigate the harmful effects of excess fat accumulation.
The next steps in this research will likely involve further investigation into how cholesterol homeostasis can be modulated in Tregs and whether such modulation can be translated into clinical therapies. Additionally, studies in human populations will be essential to confirm the relevance of these findings in people with obesity and to identify potential biomarkers that could guide treatment strategies.
Ultimately, this research underscores the complex and multifaceted nature of obesity as a disease, one that involves not just the accumulation of excess fat but also profound disruptions in immune function and metabolic regulation. By targeting the immune system, specifically the role of Tregs in fat tissue, scientists may have uncovered a new frontier in the battle against obesity and its related diseases. With continued research and innovation, these findings have the potential to transform how we treat one of the most pressing health challenges of our time.
Reference: Cody Elkins et al, Obesity reshapes regulatory T cells in the visceral adipose tissue by disrupting cellular cholesterol homeostasis, Science Immunology (2025). DOI: 10.1126/sciimmunol.adl4909