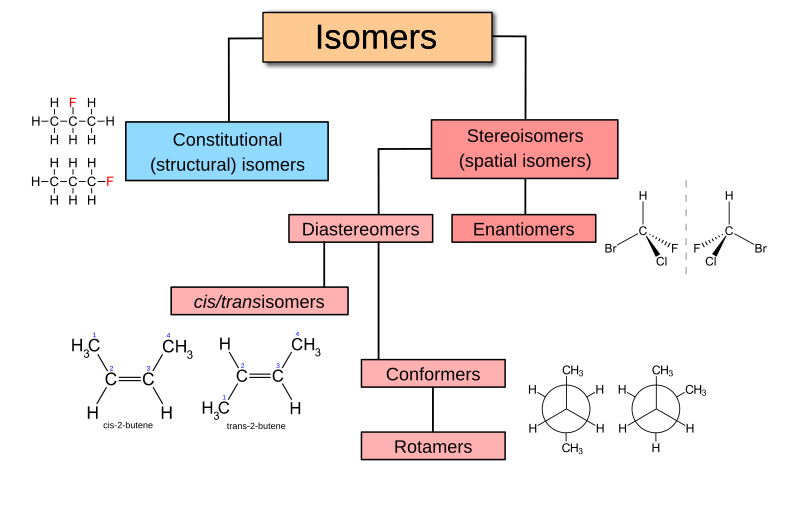
Organic chemistry is the branch of chemistry that deals with carbon-based compounds, which are the fundamental building blocks of life. From the food we eat to the medicine we rely on, organic compounds are essential in nearly every aspect of our lives. What makes these compounds unique and recognizable are their functional groups—specific groups of atoms within molecules that are responsible for the characteristic reactions of those molecules.
Understanding functional groups is crucial because they determine the chemical behavior of a compound. Whether you’re synthesizing a new drug or designing a polymer, functional groups are the key to predicting and controlling chemical reactions. In this article, we’ll explore the top 10 functional groups in organic chemistry, examining their structures, properties, and reactivity.
What Are Functional Groups?
In organic chemistry, a functional group refers to a group of atoms in a molecule that is responsible for the chemical properties and reactions of that molecule. Functional groups are the part of the molecule that interacts with other compounds and can undergo specific reactions, making them central to the behavior of organic compounds. By recognizing and understanding functional groups, chemists can predict how a molecule will react under certain conditions.
Functional groups are often classified by the types of atoms they contain, and each class of functional group exhibits certain characteristic behaviors. These functional groups are the same across different molecules, meaning that the presence of a particular functional group will always lead to certain types of chemical reactions, regardless of the size or complexity of the molecule.
The Importance of Functional Groups
Functional groups are essential in determining the chemical properties of organic compounds, including their reactivity, solubility, and polarity. For example, alcohols have a hydroxyl group (-OH) that makes them polar and able to engage in hydrogen bonding, affecting their boiling points and solubility in water. Similarly, carboxylic acids contain a carboxyl group (-COOH) that allows them to donate protons (H+), giving them acidic properties.
In addition to their role in organic chemistry, functional groups are also integral in areas such as biochemistry, pharmacology, and materials science. The structure-activity relationships in drug design, for example, rely heavily on the understanding of functional groups. By modifying the functional groups in a molecule, scientists can enhance or alter the molecule’s biological activity, making functional groups an indispensable tool in research and development.
1. Alcohols: The Hydroxyl Group (-OH)
Alcohols are one of the most common and versatile functional groups in organic chemistry. The defining feature of alcohols is the hydroxyl group (-OH), which consists of an oxygen atom bonded to a hydrogen atom. This functional group is attached to a carbon atom, which may be part of an alkyl chain or an aromatic ring.
Structure and Properties of Alcohols
The general formula for alcohols is R-OH, where “R” represents an alkyl or aryl group. The presence of the hydroxyl group makes alcohols polar and capable of forming hydrogen bonds, which significantly affects their physical properties. Alcohols typically have higher boiling points than hydrocarbons of similar molecular weight due to the ability to form hydrogen bonds. They are also generally more soluble in water than nonpolar compounds because of their polarity.
Alcohols can be classified into three categories:
- Primary Alcohols: The hydroxyl group is attached to a carbon atom that is bonded to only one other carbon atom.
- Secondary Alcohols: The hydroxyl group is attached to a carbon atom that is bonded to two other carbon atoms.
- Tertiary Alcohols: The hydroxyl group is attached to a carbon atom that is bonded to three other carbon atoms.
Reactivity of Alcohols
Alcohols are reactive compounds and can undergo various reactions depending on the conditions and the type of alcohol. Some common reactions include:
- Oxidation: Alcohols can be oxidized to aldehydes, ketones, or carboxylic acids. The type of alcohol (primary, secondary, or tertiary) determines the product formed during oxidation.
- Dehydration: Alcohols can undergo dehydration (loss of water) to form alkenes, a process catalyzed by acids.
- Esterification: Alcohols react with carboxylic acids to form esters, a reaction that is important in the production of fragrances, flavors, and plastics.
2. Aldehydes: The Carbonyl Group (-C=O)
Aldehydes are organic compounds that contain a carbonyl group (C=O), where the carbon atom is double-bonded to an oxygen atom. The carbonyl group is bonded to a hydrogen atom and an alkyl or aryl group. Aldehydes are often characterized by their distinct odors, which can range from pleasant (such as in vanilla) to pungent (such as in formaldehyde).
Structure and Properties of Aldehydes
The general formula for aldehydes is R-CHO, where “R” can be a hydrogen atom or a carbon-containing group. The carbonyl group imparts a high degree of polarity to the molecule, which influences its solubility and reactivity. Aldehydes tend to be more reactive than ketones due to the presence of the hydrogen atom attached to the carbonyl group, making them more susceptible to nucleophilic attack.
Aldehydes typically have low boiling points compared to alcohols because they cannot form hydrogen bonds. However, their boiling points are higher than hydrocarbons of similar size due to the dipole-dipole interactions between the carbonyl group and other polar molecules.
Reactivity of Aldehydes
Aldehydes are highly reactive compounds and undergo a variety of reactions, including:
- Reduction: Aldehydes can be reduced to primary alcohols by adding hydrogen or using reducing agents like lithium aluminum hydride (LiAlH4).
- Oxidation: Aldehydes can be oxidized to carboxylic acids. The hydrogen atom attached to the carbonyl carbon makes aldehydes more easily oxidized than ketones.
- Nucleophilic Addition: Aldehydes are subject to nucleophilic addition reactions, where a nucleophile (such as an alcohol or amine) adds to the carbonyl carbon.
Aldehydes play a key role in organic synthesis and are important intermediates in the production of many chemicals, including fragrances, dyes, and pharmaceuticals.
3. Ketones: The Carbonyl Group with Two Alkyl Groups
Like aldehydes, ketones also contain a carbonyl group (C=O), but the key difference is that the carbonyl carbon in ketones is bonded to two alkyl or aryl groups rather than a hydrogen atom. Ketones are common in organic chemistry and are found in many natural products, including sugars and hormones.
Structure and Properties of Ketones
The general formula for ketones is R-CO-R’, where “R” and “R'” are alkyl or aryl groups. Because the carbonyl group is surrounded by carbon atoms, ketones tend to be less reactive than aldehydes, which makes them more stable. However, ketones still exhibit many of the same physical and chemical properties as aldehydes due to the presence of the polar carbonyl group.
Ketones are typically more soluble in water than hydrocarbons due to their polarity, but they are less soluble than alcohols because they cannot form hydrogen bonds with water as effectively as alcohols. Ketones also have relatively low boiling points compared to alcohols, though higher than those of hydrocarbons of similar molecular weight.
Reactivity of Ketones
Ketones are also reactive compounds and participate in many of the same reactions as aldehydes:
- Reduction: Ketones can be reduced to secondary alcohols using reducing agents like LiAlH4 or sodium borohydride (NaBH4).
- Nucleophilic Addition: Ketones undergo nucleophilic addition reactions, similar to aldehydes, where a nucleophile adds to the carbonyl carbon.
- Aldol Condensation: Ketones can undergo aldol condensation reactions, where two molecules of a ketone react in the presence of a base to form a β-hydroxy ketone or an α,β-unsaturated ketone.
Ketones are found in many everyday products, such as acetone (a common solvent) and methyl ethyl ketone (used in paints and coatings). Their reactivity makes them valuable intermediates in organic synthesis.
4. Carboxylic Acids: The Carboxyl Group (-COOH)
Carboxylic acids are organic compounds that contain a carboxyl group (-COOH), which consists of a carbonyl group (C=O) attached to a hydroxyl group (-OH). This functional group imparts acidic properties to the compound, and carboxylic acids are known for their ability to donate protons (H+).
Structure and Properties of Carboxylic Acids
The general formula for carboxylic acids is R-COOH, where “R” represents an alkyl or aryl group. The carboxyl group makes carboxylic acids highly polar and capable of forming hydrogen bonds with water and other compounds. As a result, carboxylic acids typically have higher boiling points than alcohols or aldehydes of similar size.
Carboxylic acids are also more soluble in water than many other organic compounds because of their ability to form hydrogen bonds. Their solubility decreases as the carbon chain increases, as the nonpolar alkyl group becomes more dominant.
Reactivity of Carboxylic Acids
Carboxylic acids are relatively reactive and undergo a variety of chemical reactions, including:
- Acid-Base Reactions: Carboxylic acids can donate a proton (H+) to form a carboxylate anion (R-COO-), making them weak acids. This ability is crucial in many biological processes.
- Esterification: Carboxylic acids react with alcohols to form esters, which are often used in perfumes, food flavorings, and plastics.
- Reduction: Carboxylic acids can be reduced to primary alcohols by using reducing agents like LiAlH4.
The carboxyl group plays a critical role in many biochemical processes, including the synthesis of amino acids, fatty acids, and many important drugs and biomolecules.
5. Esters: The Ester Group (-COO-)
Esters are organic compounds that contain a carbonyl group (C=O) bonded to an oxygen atom, which is further bonded to another carbon atom. The structure of esters is derived from carboxylic acids, where the hydroxyl group (-OH) has been replaced by an alkoxy group (-OR). Esters are responsible for the characteristic smells of many fruits and flowers, making them key players in the fragrance and flavor industry.
Structure and Properties of Esters
The general formula for esters is R-COO-R’, where “R” and “R'” are alkyl or aryl groups. The ester functional group imparts a unique set of properties to these compounds. Esters are typically less polar than carboxylic acids and alcohols, meaning they have lower boiling points. This makes esters volatile and fragrant, which is why they are often used in perfumes and flavorings.
Esters are usually quite soluble in organic solvents, but their solubility in water decreases as the size of the alkyl group increases. Smaller esters, like methyl acetate, are more soluble in water compared to larger esters.
Reactivity of Esters
Esters are relatively reactive and undergo several key reactions:
- Hydrolysis: In the presence of water and an acid or base catalyst, esters can undergo hydrolysis, breaking down into a carboxylic acid and an alcohol. This process is often used in soap making, where triglycerides (esters of glycerol) are hydrolyzed into fatty acids and glycerol.
- Transesterification: Esters can undergo transesterification, a reaction where one ester reacts with an alcohol to form a different ester.
- Reduction: Esters can be reduced to alcohols using reducing agents like lithium aluminum hydride (LiAlH4).
Esters are ubiquitous in both nature and industry. They are used extensively in food flavorings, fragrances, plastics, and pharmaceuticals, highlighting their versatility in a wide array of applications.
6. Amines: The Amino Group (-NH2)
Amines are organic compounds that contain a nitrogen atom bonded to one or more carbon atoms. The nitrogen atom in amines has a lone pair of electrons, making amines basic in nature. Amines are classified based on the number of alkyl or aryl groups attached to the nitrogen atom. Primary amines have one alkyl group attached to the nitrogen, secondary amines have two, and tertiary amines have three.
Structure and Properties of Amines
The general formula for amines is R-NH2, R2NH, or R3N, depending on the number of alkyl groups attached to the nitrogen. Amines can be classified as aliphatic (when the nitrogen is attached to carbon chains) or aromatic (when the nitrogen is attached to aromatic rings). The lone pair of electrons on the nitrogen atom makes amines basic and capable of accepting protons (H+), which gives them alkaline properties.
Amines have relatively high boiling points compared to hydrocarbons, as they can form hydrogen bonds with other molecules. However, their boiling points are lower than those of alcohols, due to the lower polarity of the nitrogen-hydrogen bond compared to the oxygen-hydrogen bond in alcohols.
Reactivity of Amines
Amines undergo a variety of chemical reactions, especially as bases:
- Acid-Base Reactions: Amines can act as bases, accepting protons to form ammonium salts (R-NH3+). This is important in the formation of amine salts, which are used in drug formulations.
- Nucleophilic Substitution: Amines can undergo nucleophilic substitution reactions, where the lone pair on nitrogen attacks an electrophilic carbon, replacing a leaving group.
- Formation of Amides: Amines can react with carboxylic acids or their derivatives (like acyl chlorides) to form amides, which are key intermediates in protein synthesis.
Amines are fundamental in both organic chemistry and biochemistry, as they are found in many important biomolecules, including amino acids, neurotransmitters, and alkaloids. Additionally, amines are used in the production of dyes, pharmaceuticals, and agrochemicals.
7. Amides: The Amido Group (-CONH2)
Amides are organic compounds that contain a carbonyl group (C=O) bonded to a nitrogen atom. They are derived from carboxylic acids, where the hydroxyl group (-OH) is replaced by an amine group (-NH2). Amides are often found in proteins and peptides, where they form the backbone of these vital biological molecules. The functional group is also found in many polymers, such as nylon.
Structure and Properties of Amides
The general formula for amides is R-CO-NH2, where “R” is an alkyl or aryl group. The amide functional group is characterized by its strong polar nature, which allows amides to engage in hydrogen bonding. This makes amides generally have higher boiling points than esters, ketones, and aldehydes of similar size. They also tend to be relatively stable compounds and are less reactive than their carboxylic acid precursors.
Amides are typically soluble in polar solvents, but their solubility in water decreases as the size of the alkyl group increases.
Reactivity of Amides
Amides undergo several types of reactions, most notably:
- Hydrolysis: Amides can be hydrolyzed in the presence of acids or bases to form a carboxylic acid and an amine. This reaction is particularly important in biological processes, such as the breakdown of proteins.
- Reduction: Amides can be reduced to amines using powerful reducing agents, like lithium aluminum hydride (LiAlH4).
- Nucleophilic Substitution: Amides can also undergo nucleophilic substitution reactions, where a nucleophile replaces the amide nitrogen in the molecule.
The presence of the amide group makes these compounds crucial in many biological and industrial applications. They are key components of peptides, proteins, and synthetic polymers, such as nylons and polyurethanes.
8. Nitriles: The Nitrile Group (-C≡N)
Nitriles are organic compounds that contain a cyano group (C≡N), where a carbon atom is triple-bonded to a nitrogen atom. Nitriles are often found in organic synthesis and play an important role as intermediates in the production of other functional groups. The cyano group is highly polar, making nitriles reactive compounds in nucleophilic substitution and addition reactions.
Structure and Properties of Nitriles
The general formula for nitriles is R-C≡N, where “R” represents an alkyl or aryl group. Nitriles are relatively small and simple, but the presence of the strong triple bond between carbon and nitrogen makes them polar. This polarity influences their boiling points and solubility, with nitriles typically having higher boiling points than hydrocarbons of similar size due to their dipole-dipole interactions.
Nitriles are polar and tend to be soluble in polar solvents, but they are not very soluble in water due to their relatively low hydrogen bonding ability.
Reactivity of Nitriles
Nitriles undergo several important reactions:
- Hydrolysis: Nitriles can be hydrolyzed under acidic or basic conditions to form carboxylic acids. This is a key step in the industrial synthesis of acids like acetic acid.
- Reduction: Nitriles can be reduced to amines, which is an important reaction in the production of pharmaceuticals and agrochemicals.
- Nucleophilic Addition: Nitriles can react with nucleophiles (such as Grignard reagents or organolithium compounds) to form various functional groups like ketones or aldehydes.
Nitriles are versatile intermediates in organic synthesis, often used to prepare a wide variety of functional groups and complex organic molecules.
9. Thiols: The Sulfhydryl Group (-SH)
Thiols, also known as mercaptans, are organic compounds that contain a sulfur atom bonded to a hydrogen atom, forming the sulfhydryl group (-SH). Thiols are similar to alcohols, but instead of an oxygen atom, they have sulfur in the hydroxyl group position. The sulfur atom in thiols is larger and less electronegative than oxygen, which gives thiols distinct chemical and physical properties.
Structure and Properties of Thiols
The general formula for thiols is R-SH, where “R” represents an alkyl or aryl group. Thiols are polar molecules, but their polarity is not as strong as that of alcohols. Thiols tend to have a strong, often unpleasant odor, which is why they are commonly found in compounds like natural gas (to add a detectable odor) and in some biological systems, such as in the structure of proteins.
Thiols have lower boiling points than alcohols of similar size, but they are still more volatile than hydrocarbons. They can form hydrogen bonds with other thiol molecules, but these interactions are weaker than the hydrogen bonds formed by alcohols.
Reactivity of Thiols
Thiols are reactive compounds that undergo several important reactions:
- Oxidation: Thiols can be oxidized to disulfides, which are important in stabilizing the structures of proteins.
- Substitution: Thiols can react with alkyl halides to form thioethers, which are important in organic synthesis.
- Metal Coordination: Thiols can bind to metals, forming metal-thiol complexes that play an essential role in biological systems.
Thiols are crucial in the study of biochemistry, particularly in protein chemistry, where the sulfhydryl group plays a central role in the folding and function of enzymes and other biomolecules.
10. Halides: The Halogen Group (-X)
Halides are organic compounds that contain a halogen atom (fluorine, chlorine, bromine, or iodine) bonded to a carbon atom. Halides are classified based on the type of halogen they contain, with alkyl halides being the most common. These compounds are highly reactive and serve as intermediates in a wide range of organic reactions.
Structure and Properties of Halides
The general formula for halides is R-X, where “R” is an alkyl or aryl group, and “X” represents a halogen atom. Halides are polar due to the electronegativity difference between carbon and the halogen, which makes them reactive in nucleophilic substitution reactions. The boiling points of halides vary depending on the size and electronegativity of the halogen involved.
Halides are often less soluble in water than alcohols or carboxylic acids due to their inability to form hydrogen bonds with water.
Reactivity of Halides
Halides are key in a variety of organic reactions, including:
- Nucleophilic Substitution: Halides can undergo nucleophilic substitution reactions, where the halogen atom is replaced by a nucleophile, such as an alcohol or amine.
- Elimination Reactions: Halides can participate in elimination reactions, where a halogen atom and a hydrogen atom are removed from adjacent carbon atoms to form a double bond (alkene).
- Radical Reactions: Halides can undergo radical reactions, where the halogen atom is replaced by a different atom or group, often seen in polymerization processes.
Halides are essential in organic synthesis, especially in the preparation of pharmaceuticals, plastics, and agrochemicals.
Conclusion
Functional groups are the cornerstone of organic chemistry. By understanding the behavior of these groups—whether it’s the alcohol hydroxyl group (-OH) or the carbonyl group (-C=O) in aldehydes and ketones—chemists can predict the reactivity and properties of molecules. This knowledge is not just foundational for academic study; it has real-world applications in drug development, materials science, and environmental chemistry.
In this article, we have explored the top 10 functional groups that shape organic chemistry. From the common hydroxyl group in alcohols to the reactive halide groups, each functional group offers unique insights into molecular behavior and reactivity. By learning how these functional groups interact and react with other molecules, chemists can innovate and create new compounds with desired properties, enhancing industries such as pharmaceuticals, agriculture, energy, and manufacturing.
Understanding these functional groups provides a framework to explore even more complex reactions and compounds. As science advances, the potential for new discoveries and applications of these functional groups only continues to grow, making them as crucial today as they have ever been.