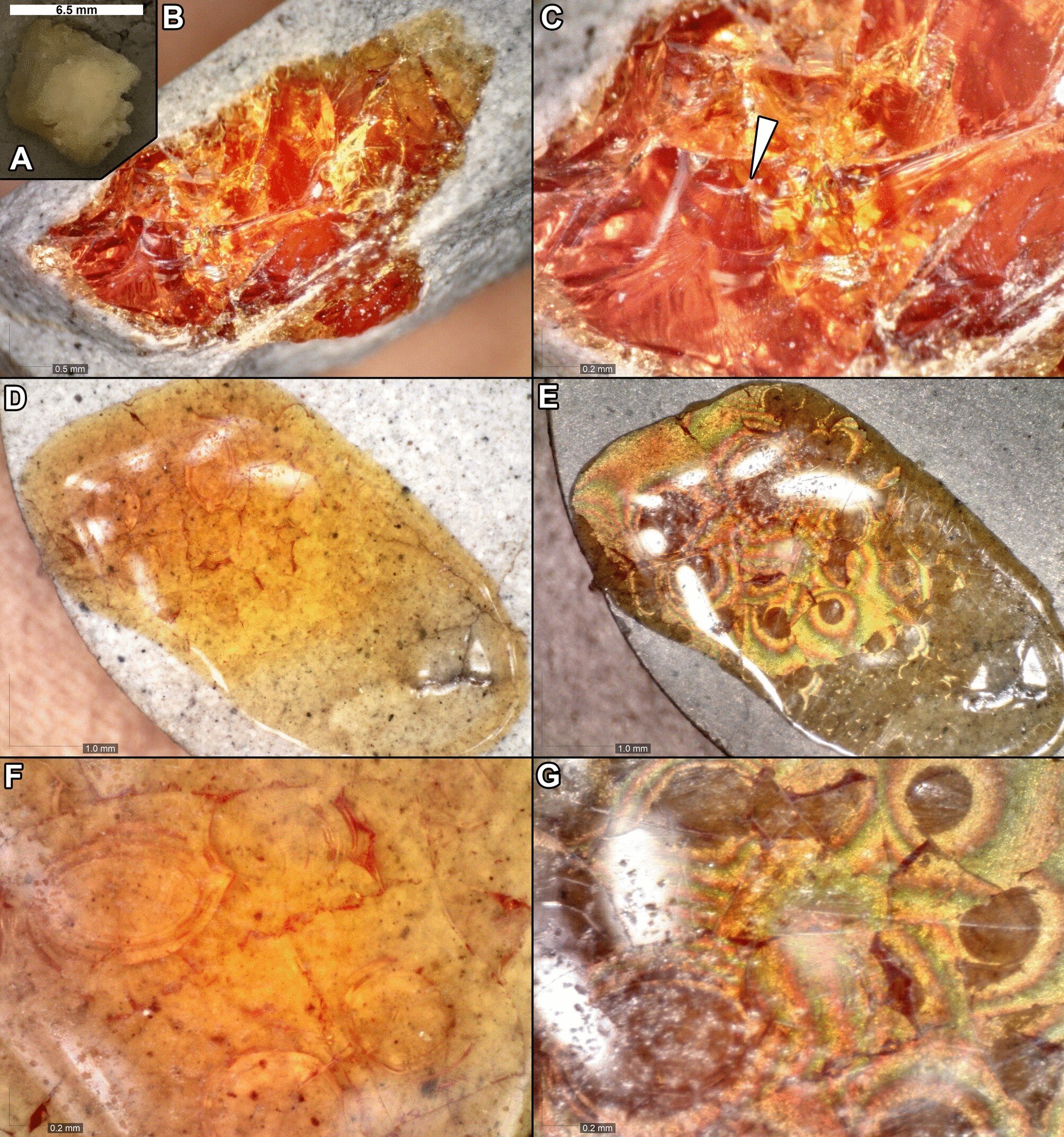
Amber and copal have fascinated scientists and collectors alike for centuries. These fossilized tree resins, often found encasing prehistoric insects and plant fragments, serve as pristine time capsules, preserving delicate specimens for millions of years. However, the rarity and scientific importance of amber inclusions make destructive testing virtually impossible. Now, a groundbreaking study by the Field Museum in Chicago and the Foundation for Scientific Advancement has taken a major step forward in unraveling the mysteries of fossil resin formation. By simulating the natural maturation of tree resin under controlled conditions, researchers have successfully created a hardened, translucent substance that closely mimics natural copal and amber—paving the way for a new era of amber research.
The Challenge of Studying Amber and Copal
Amber and its younger counterpart, copal, provide an invaluable window into prehistoric ecosystems. They preserve the fine details of insects, pollen grains, and plant tissues, offering rare insights into ancient life. However, these fossils are not just visually stunning—they are chemically complex. Studying their composition often requires advanced analytical techniques, but traditional chemical analysis is destructive, permanently altering or destroying the precious specimens in the process.
For years, scientists have sought ways to understand amber’s chemical evolution while minimizing damage to rare fossil samples. The ability to replicate amber in a laboratory setting could be a game-changer, allowing researchers to develop and test new methods on synthetic specimens before applying them to genuine amber. But until now, attempts to replicate amber-like resin under realistic geological conditions have fallen short.
Simulating the Fossilization of Resin
In the study, Experimental Maturation of Pine Resin in Sediment to Investigate the Formation of Synthetic Copal and Amber, published in Scientific Reports, scientists tackled the challenge head-on. They aimed to simulate the natural transformation of fresh pine resin into fossilized resin analogs under controlled conditions.
The team collected resin samples from a Mountain Pine (Pinus mugo) and two Scots Pines (Pinus sylvestris) at the Chicago Botanic Garden. To mimic natural burial conditions, the resin was encased in bentonite clay—a type of fine-grained sediment that could act as a natural trapping medium, similar to the sediments that entomb resin in nature.
The samples were then subjected to high pressure (ranging from 159 to 241 bar) and elevated temperatures (130°C to 150°C) for extended periods (19 to 41 hours). These conditions were designed to accelerate the geological processes that typically take thousands to millions of years.
Observing the Transformation: From Sticky Sap to Fossilized Resin
The effects of the experimental treatment were striking. Initially, the fresh resin was soft, sticky, and opaque, but after exposure to heat and pressure, it transformed into a brittle, translucent material with a deep amber-like coloration. Digital light microscopy revealed conchoidal fracturing—a pattern of breakage commonly seen in natural copal and amber—along with birefringence under cross-polarized light, flow lines, and internal air pockets.
These visual similarities to natural amber were promising, but the researchers needed to confirm whether the chemical changes mirrored those found in true fossilized resin.
Chemical Analysis: A Step Closer to True Amber
To assess the chemical transformation, the scientists used infrared spectroscopy, a technique that identifies molecular structures based on how they absorb infrared light. The spectral data revealed a significant reduction in the carbonyl (C=O) signal at 1700 cm⁻¹—an important chemical marker also observed in natural copal and Eocene Baltic amber.
This shift suggested that the experimental resin had undergone processes similar to those in natural fossilization, including the loss of volatile terpenoid compounds and the polymerization of remaining diterpenoid components. In nature, these changes help transform soft resin into the hard, glassy substance we recognize as amber.
Crucially, the lab-grown resin also demonstrated the movement of volatile components into the surrounding clay, mimicking natural resin maturation. This result confirmed that sediment plays an essential role in the fossilization process, absorbing and trapping chemical byproducts as the resin stabilizes over time.
The Future of Synthetic Amber Research
While the study represents a major breakthrough, the researchers acknowledge that their synthetic resin is not yet an exact chemical match to true amber. Some aspects of polymerization—the process that gives amber its characteristic hardness and resistance to decay—may still differ from natural fossilized resin.
To refine the process, future experiments will focus on fine-tuning temperature and pressure conditions, as well as testing resins from different tree species. Certain trees, such as Agathis and Hymenaea, produce resins rich in polymerizable diterpenoids, making them ideal candidates for further study.
By developing an amber analog that closely replicates the real thing, scientists hope to revolutionize how we study fossilized resins. A laboratory-made amber substitute would allow for rigorous testing of chemical analysis techniques without sacrificing rare fossil specimens. This could lead to better methods for identifying ancient amber sources, tracking changes in prehistoric ecosystems, and even detecting forgeries in the amber trade.
Conclusion: A New Frontier in Paleontology
The ability to synthesize amber-like resin in the lab marks an exciting step forward in paleontology and materials science. By recreating the fossilization process under controlled conditions, scientists have opened new doors for the study of ancient resins, their inclusions, and their chemical evolution.
As research continues, the creation of synthetic amber could unlock new ways to explore Earth’s deep history, helping us decode the secrets trapped within these golden relics of the past. Whether for scientific discovery, conservation, or even industry applications, this breakthrough paves the way for a deeper understanding of one of nature’s most fascinating materials.
Reference: Evan T. Saitta et al, Experimental maturation of pine resin in sediment to investigate the formation of synthetic copal and amber, Scientific Reports (2025). DOI: 10.1038/s41598-025-89448-5